Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1072
Peer-review started: December 2, 2017
First decision: December 27, 2017
Revised: January 2, 2018
Accepted: January 16, 2018
Article in press: January 16, 2018
Published online: March 14, 2018
To investigate the response to hyperthermia and chemotherapy, analyzing apoptosis, cytotoxicity, and cisplatin concentration in different digestive system cancer cells.
AGS (gastric cancer cell line), Caco-2 (colon cancer cell line) and T3M4 (pancreatic cancer cell line) were treated by cisplatin and different temperature setting (37 °C to 45 °C) either in isolation, or in combination. Treatment lasted for one hour. 48 h after the treatment viability was evaluated by MTT, cell apoptosis by Annexin V-PE and 7ADD flow cytometry. Intracellular cisplatin concentration was measured immediately after the treatment, using mass spectrometry. Isobologram analysis was performed to evaluate the mathematical combined effect of temperature and cisplatin.
AGS cells were the most sensitive to isolated application of hyperthermia. Hyperthermia, in addition to cisplatin treatment, did not provoke a synergistic effect at intervals from 37 °C to 41 °C in neither cancer cell line. However, a temperature of 43 °C enhanced cisplatin cytotoxicity for Caco-2 cells. Moreover, isobologram analysis revealed mathematical antagonistic effects of cisplatin and temperature combined treatment in AGS cells; variations between synergistic, additive, and antagonistic effects in Caco-2 cells; and additive and antagonistic effects in T3M4 cells. Combined treatment enhanced initiation of cell apoptosis in AGS, Caco-2, and T3M4 cells by 61%, 20%, and 19% respectively. The increase of intracellular cisplatin concentration was observed at 43 °C by 30%, 20%, and 18% in AGS, Caco-2, and T3M4 cells, respectively.
In addition to cisplatin, hyperthermia up to 43 °C does not affect the viability of cancer cells in a synergistic manner.
Core tip: Hyperthermal intraperitoneal chemotherapy is widely used as a standard treatment option for peritoneum invading gastrointestinal cancer. Our in vitro results suggest that optimal temperature has to be taken into consideration for achieving optimal therapeutic effect. In addition to cisplatin, hyperthermia up to 43 °C does not affect the viability of AGS, Caco-2, and T3M4 cells in a synergistic manner. However, some regimens of hyperthermia and cisplatin treatment are beneficial regarding an increase in intracellular cisplatin concentration and enhancement apoptosis of gastrointestinal cancer cells.
- Citation: Cesna V, Sukovas A, Jasukaitiene A, Naginiene R, Barauskas G, Dambrauskas Z, Paskauskas S, Gulbinas A. Narrow line between benefit and harm: Additivity of hyperthermia to cisplatin cytotoxicity in different gastrointestinal cancer cells. World J Gastroenterol 2018; 24(10): 1072-1083
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1072
For the past two decades, hyperthermal intraperitoneal chemotherapy (HIPEC) has been considered as a treatment option for peritoneum invading gastrointestinal cancers[1]. Various studies have demonstrated improved survival rates for gastric[2] and colorectal cancers[3-5]. The clinical application of hyperthermia is based on the assumption that it may enhance the effect of the chemotherapy, especially cisplatin-based treatments[6-8]. There are some experimental studies providing evidence that hyperthermia can affect cell membranes, cytoskeletons, synthesis of macromolecules, increase drug-induced DNA damage, and inhibit the repair of drug-induced DNA damage[9]. Hyperthermia may provide higher local cisplatin concentrations in tissues, indicating the pharmacokinetic advantage of its use and reduction of systemic toxicity[10]. Hyperthermia-induced PARP blockade can increase chemotherapy-induced damage in BRCA-competent cells of ovarian and colon cancer[11].
However, the results of available studies on the synergy of hyperthermia and cisplatin chemotoxicity, initiation of apoptosis, and intracellular accumulation of cisplatin in different gastrointestinal cancer cells are controversial. The opposite effect of hyperthermia on cisplatin sensitivity was observed in mismatch repair deficiency and mismatch repair proficiency in colon cancer cell lines[12]. Isolated hyperthermia only temporarily inhibited cell proliferation without cytotoxic effects on gastric cancer cell lines. However, a synergistic effect of hyperthermia and chemotherapy on inhibiting proliferation and induction of cell death via the apoptotic pathway was reported[13]. Interestingly, the hyperthermia-mediated increase of cellular accumulation of cisplatin and persistent DNA damage in gastric cancer cells was observed only with the addition of tumor necrosis factor[14]. The expression of heat shock genes and proteins provides an adaptive mechanism for stress tolerance, allowing cells to survive non-physiologic conditions. However, the same adaptive mechanism can ultimately favor malignant transformation by interfering with pathways that regulate cell growth and apoptosis. Cytoprotection and thermotolerance raised the concern that heat-treated tumor cells might also be resistant to attack by immune effector mechanisms[15]. Data on the additive effect of hyperthermia in terms of enhanced chemo-cytotoxicity in cancer cells of pancreatic origin are scarce.
Therefore, the aim of this study was to analyze the additivity of hyperthermia to cisplatin effects in gastric, pancreatic, and colorectal cancer cell lines evaluating cell cytotoxicity, apoptosis, and intracellular cisplatin concentration.
The AGS and Caco-2 cell lines were purchased from American Type Cell Culture (ATCC Manassas, VA, United States). AGS cell line is derived from a gastric adenocarcinoma of the stomach of a 54 year-old Caucasian female with no prior anti-cancer treatment. Caco-2 cells were isolated from a primary colonic tumor in a 72-year-old Caucasian male using the explant culture technique. Forms moderately well differentiated adenocarcinomas consistent with colonic primary grade II, in nude mice. T3M4 cell line was obtained as a gift from the European Pancreas Center (Heidelberg, Germany). This cell line was derived from a lymph node metastasis of the Japanese male patient, diagnosed with pancreatic ductal adenocarcinoma. It is characterized as pancreatic adenocarcinoma producing CEA, K-ras activated, and with slow cell growth. Cells were grown in RPMI medium (Gibco/Invitrogen, Carlsbad, CA, United States) with the addition of 10% fetal bovine serum (Gibco/Invitrogen) and 1% penicillin/streptomycin solution (Gibco/Invitrogen). Flasks with cells were cultured in a humid incubator with a CO2 level of 5% and temperature of 37 °C.
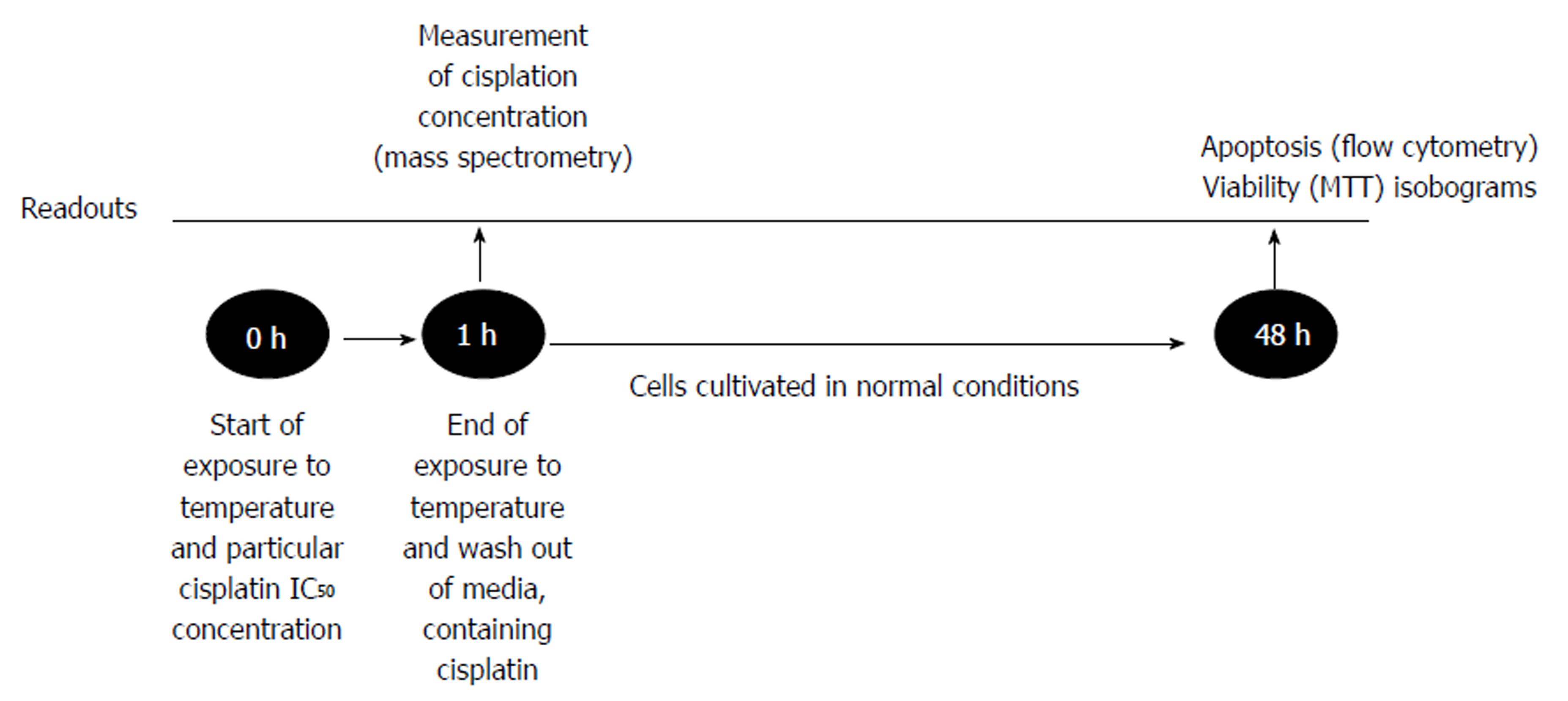
Cancer cells were cultivated for 24 h in the conditions described above. Afterwards, cells were treated by one of two separate factors: temperature (37 °C, 38 °C, 39 °C, 40 °C, 41 °C, 42 °C, 43 °C, 44 °C, 45 °C) or IC50 dose of cisplatin, which was specifically determined for each cell line. Moreover, the combination of hyperthermia and cisplatin treatment was applied (Figure 1). The duration of treatment was 1 h as it reflected the treatment time under the clinical conditions of HIPEC. Cells were heated in humid incubators at particular temperature regimens. When temperature of the media reached desired level, we started the countdown of one hour exposure. Medium temperature was controlled by the electronic thermometer. Following the treatment, the medium was changed, and cells were cultivated for 48 h under the conditions as previously described. Afterwards, MTT and flow cytometry were performed (see “MTT assay” and “Cell apoptosis”). All experiments were repeated at least three times.
Cytotoxicity of cisplatin or/and hyperthermia was determined by MTT [3-(4, 5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazolium bromide] (Gibco/Invitrogen) assay. The plate was incubated for 4 h at 37 °C after 5 mg/mL of the MTT reagent was added to the wells. Then the supernatant was removed, and DMSO (dimethyl sulfoxide) (Carl Roth GmbH, Karlsruhe, Germany) was added to solubilize the resulting formazan crystals. Absorbance measurements were made at 570 nm on the Sunrise spectrophotometer (Tecan GmbH, Grodig, Austria).
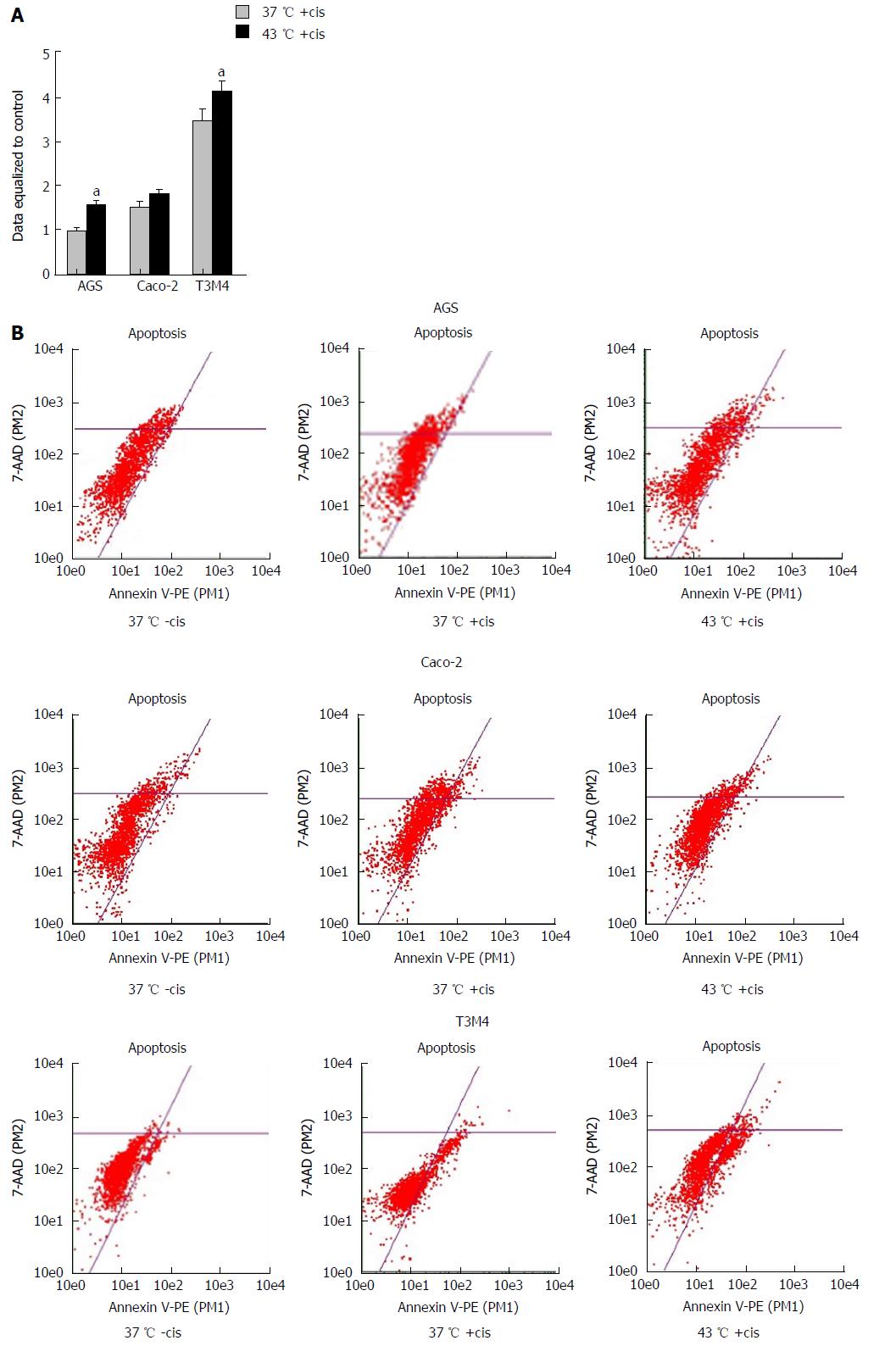
The changes in apoptosis were evaluated after treatment of the cells by hyperthermia (43 °C) and the previously determined IC50 of cisplatin (specific for particular cell line). Untreated cisplatin cells and normothermia (37 °C) cultivated cells were defined as controls. Forty-eight hours after the experiment (Figure 1), the rate of apoptosis was determined by flow cytometer Guava Nexin Annexin V Assay (Merck, Millipore United States and Canada). All the procedures were performed according to the manufacturer’s instructions. Samples were measured using a Guava Personal Cell Analysis (PCA) Flow Cytometer (Merck, Millipore) and CytoSoft software.
Immediately after the experiment, the cell lysate samples were diluted ten times with high purity deionized water. Concentration of total intracellular cisplatin was analyzed by inductively coupled plasma mass spectrometer (ICP-MS) NexION™ 300D (PerkinElmer, United States) equipped with nickel cones and a quartz cyclonic spray chamber as a sample introduction system and using collision mode kinetic energy discrimination (KED) with helium gas (purity ≥ 99.999%) to remove polyatomic interferences. The calibration graphs for determination of Platinum by ICP-MS were prepared in the range of 0-50 ng/mL using Pure Plus 10 mg/L Multi-Element calibration standard 4 (PerkinElmer, United States). The working conditions of the spectrometer were optimized daily in order to obtain the maximal sensitivity and stability.
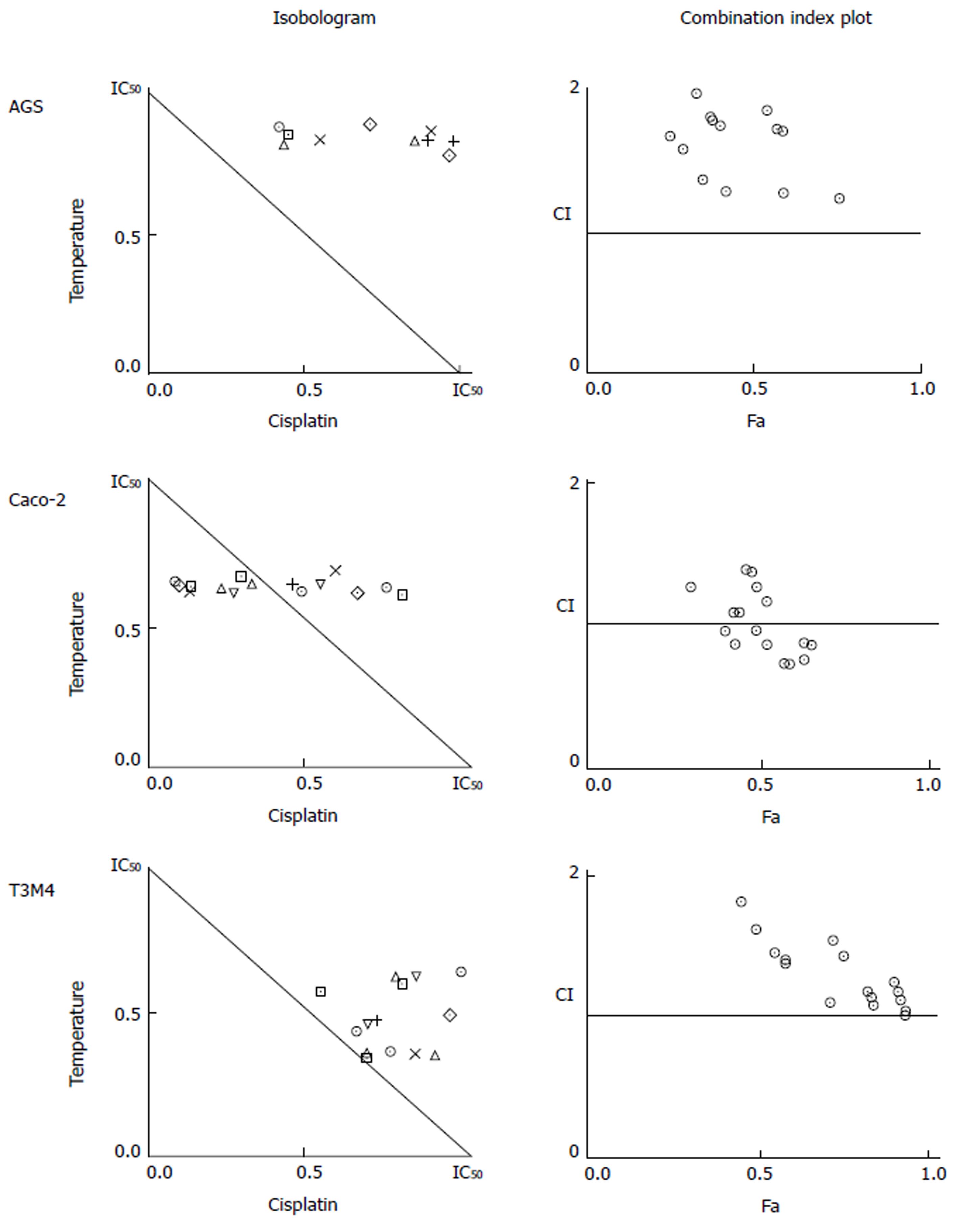
To identify the confidence limits for the additive action of hyperthermia and cisplatin, isobologram analysis was applied. Data for analysis was obtained from the MTT test. Temperature data points of 38 °C, 39 °C, 40 °C, 41 °C, 42 °C, 43 °C, 44 °C, and 45 °C, and cisplatin concentration data points of 25 μmol/L, 50 μmol/L, 100 μmol/L, 200 μmol/L, and 400 μmol/L were used to define the dose effect curve for temperature and cisplatin, respectively. For combined treatment analysis, the following combinations of factors were used: 39 °C + 50 μmol/L; 39 °C + 100 μmol/L; 39 °C + 200 μmol/L; 40 °C + 50 μmol/L; 40 °C + 100 μmol/L; 40 °C + 200 µmol/L; 41 °C + 50 μmol/L; 41 °C + 100 μmol/L; 41 °C + 200 μmol/L; 42 °C + 50 μmol/L; 42 °C + 100 μmol/L; 42 °C + 200 μmol/L; 43 °C + 50 μmol/L; 43 °C + 100 μmol/L; 43 °C + 200 μmol/L; 44 °C + 50 μmol/L; 44 °C + 100 μmol/L; 44 °C + 200 μmol/L. The Chou median effect equation was used[16]. It provides the theoretical basis for the combination index (CI), which enabled quantification of drug interaction affects defining synergism (CI < 1), additivity (CI = 1), or antagonism (CI > 1). The CompuSyn software (CompuSyn, Inc., Paramus, NJ, United States) was used for calculations.
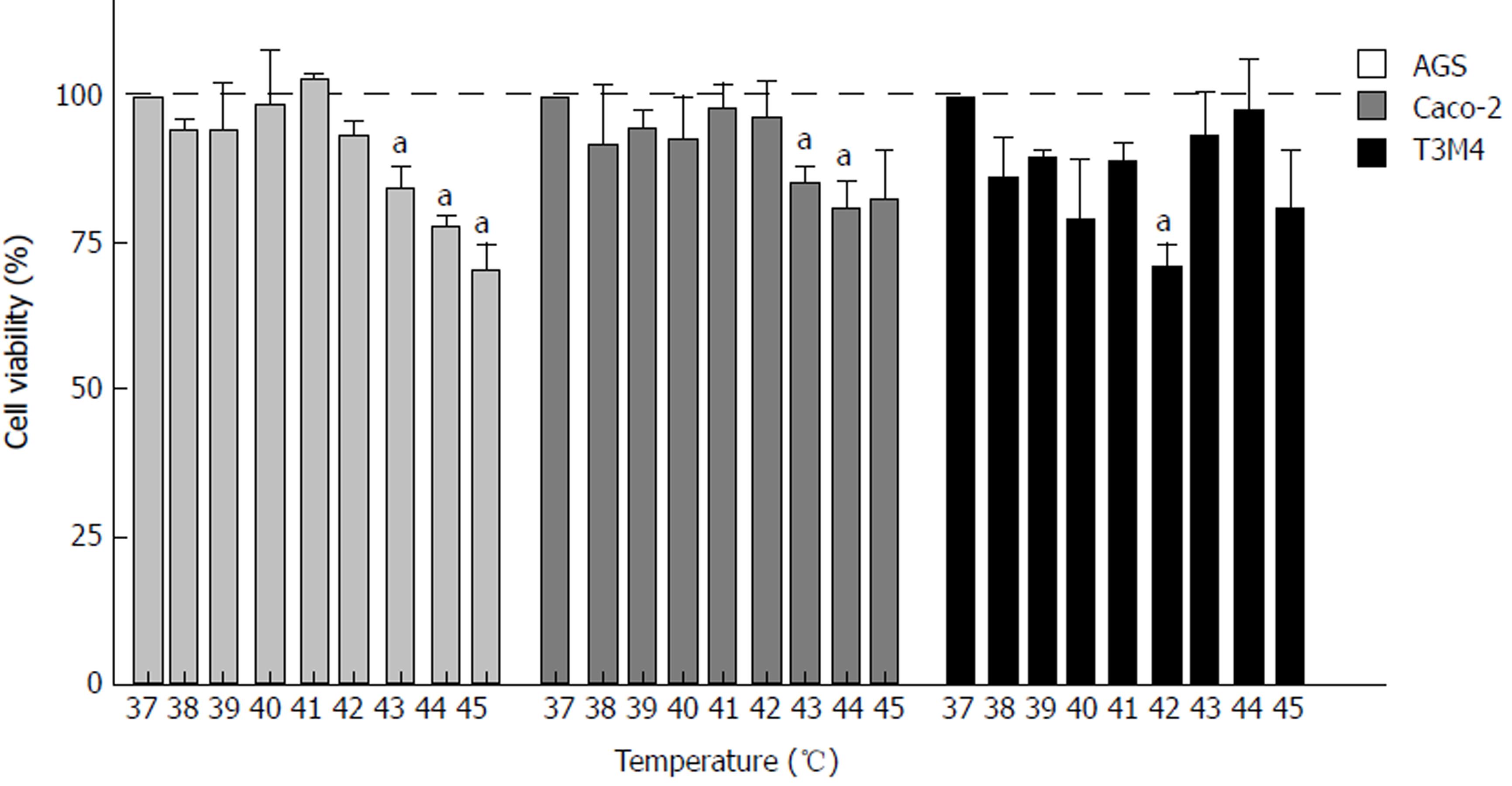
To evaluate the effect of hyperthermia per se on different cancer cells in vitro, we examined the response of the AGS, Caco-2, and T3M4 cell lines to temperature using a stepwise increase of one degree ranging from 37 °C to 45 °C. Cell viability was determined by MTT assay. Every cell line demonstrated a different pattern of response to hyperthermia (Figure 2). AGS cells were the most sensitive one to hyperthermia, where a temperature rise from 41 °C to 45 °C gradually decreased its viability by 30%. In the 37 °C to 41 °C interval, the viability stayed constant. Cells affected by 43 °C, 44 °C, and 45 °C dropped their viability rate by 16%, 22 °C, and 30%, respectively, as compared to the control (37 °C). A temperature increase from 37 °C to 42 °C had no significant effect on Caco-2 cells, but at 43 °C and 44 °C, its viability dropped by 14% and 20%, respectively, but stayed constant at higher temperatures. The response of T3M4 cells to the changes of temperature was different. Temperatures from 37 °C to 42 °C decreased its viability by 30%. At 43 °C, the viability of T3M4 cells sharply increased by 20% and stayed at this level in higher temperatures. Overall, only the AGS cell line responded to hyperthermia as implied, with gradual inhibition of cell viability.
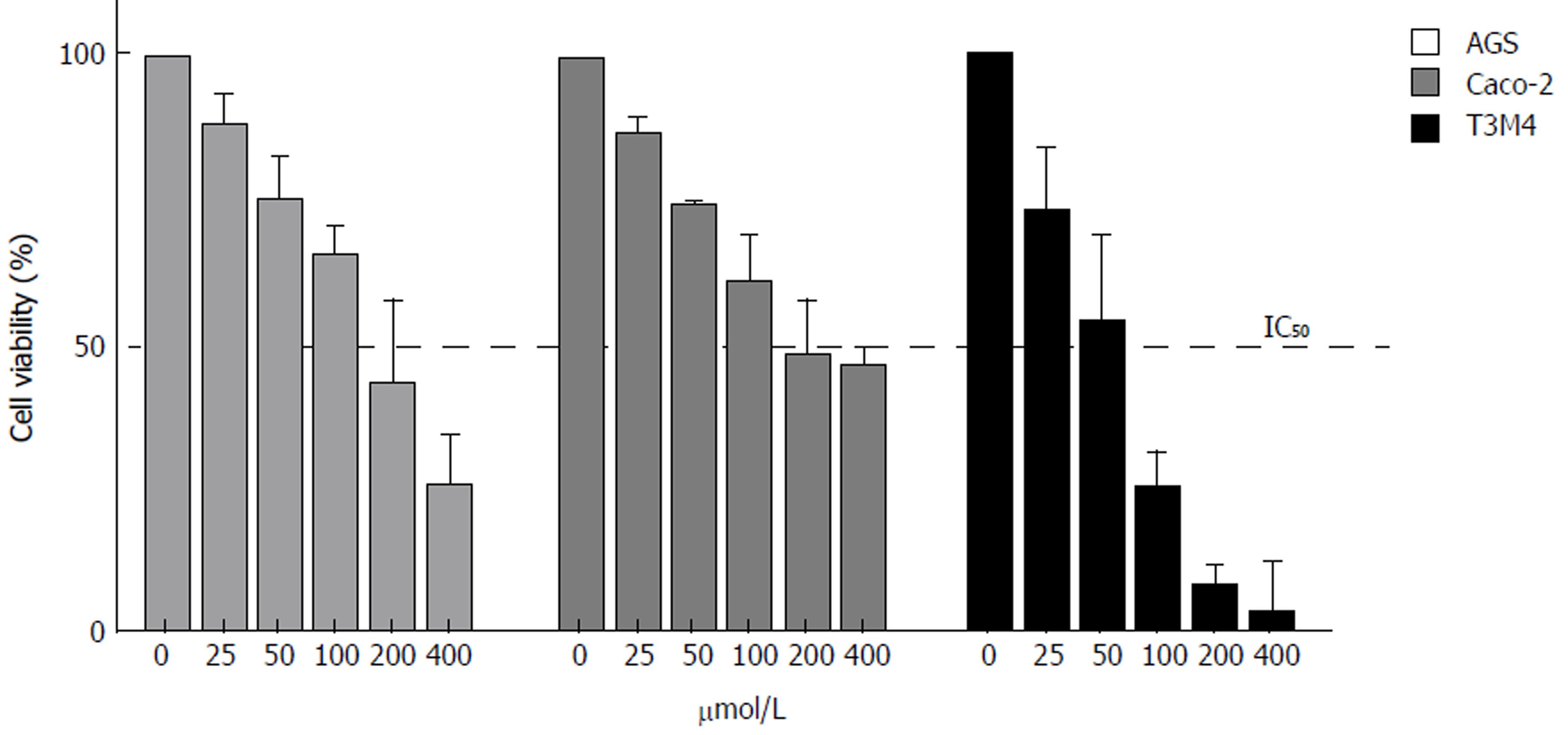
Cisplatin induced a dose-dependent suppressor effect on cancer cell viability, with a similar linear response in the AGS, Caco-2, and T3M4 cell lines. Pancreatic cancer cells (T3M4) were the most sensitive, revealing cisplatin IC50 at 48 μmol/L. The IC50 for Caco-2 was 194 μmol/L, and for AGS, it was 182 μmol/L (Figure 3).
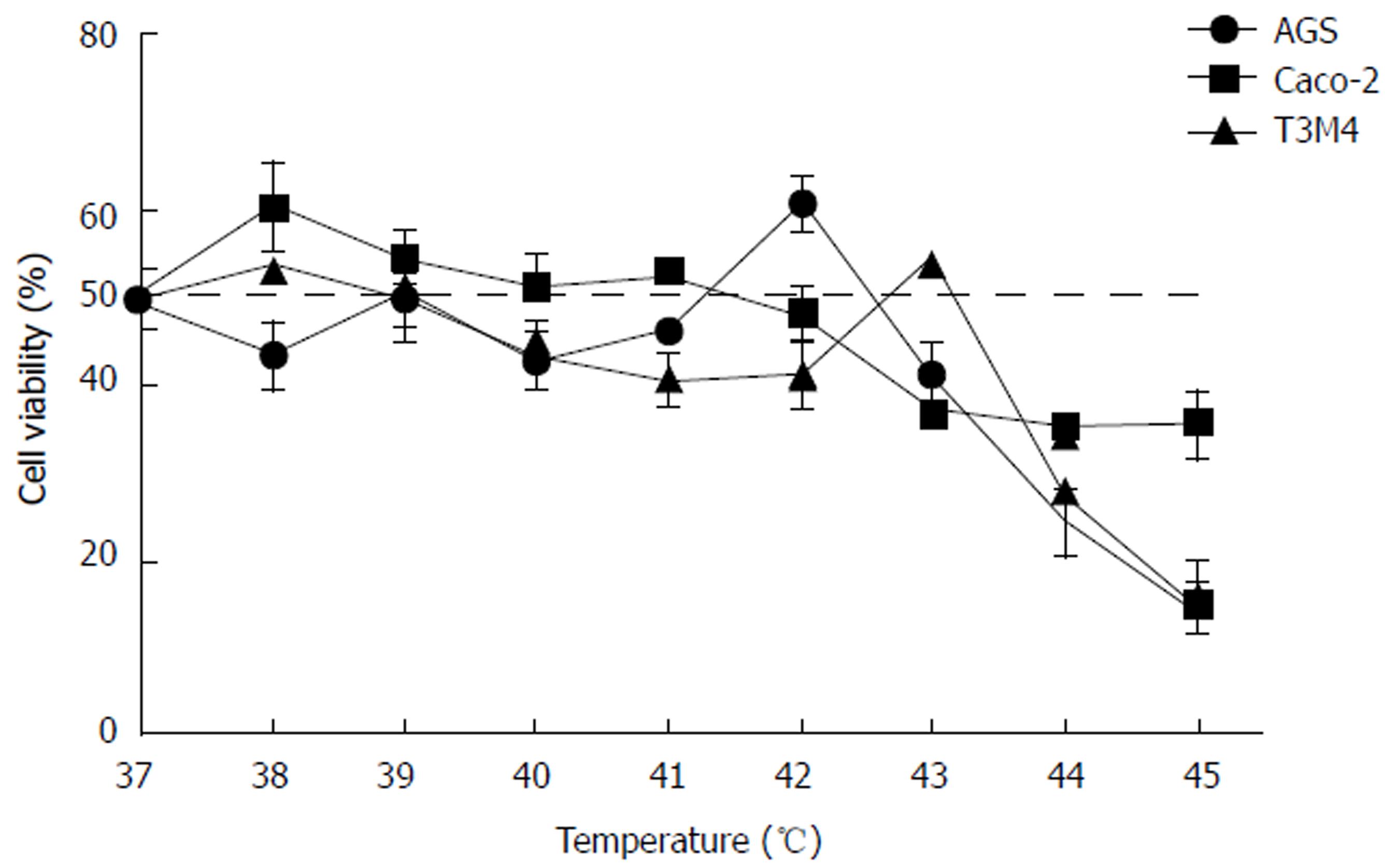
Previously determined IC50 doses of cisplatin were used for particular cell lines during this experiment. Simultaneous exposure of cells to hyperthermic conditions and cisplatin triggered different response in AGS, Caco-2, and T3M4 cells (Figure 4). Overall, temperatures ranging from 37 °C to 41 °C had no detectable additive effect to cisplatin cytotoxicity. Interestingly, we observed peaks of viability in AGS (42 °C, increase by 33%) and T3M4 (43 °C, increase by 32%) cells. Higher temperatures, in addition to cisplatin exposure, inhibited cell growth dramatically; AGS viability dropped by 70% and T3M4 dropped by 76% at 45 °C. Application of hyperthermia in addition to cisplatin in the Caco-2 cell line gradually improved the cytotoxic effect and decreased the viability of cells by one-fourth from 43 °C to 45 °C. In summary, application of hyperthermia (43 °C and higher) in addition to cisplatin might enhance its cytotoxic effect in the Caco-2 cell line. However, lower (e.g., < 43 °C) temperature regimens may even promote cell proliferation and worsen expected hyperthermal chemotherapy effects in AGS and T3M4 cell lines.
We constructed isobolograms to reveal if the combined application of hyperthermia and cisplatin had synergistic, antagonistic, or additive effects. Combination of temperature and cisplatin was strongly antagonistic for AGS cells in all studied data points. Combined application of hyperthermia and cisplatin was tripled in Caco-2 cells: synergistic (at 40 °C with 50 μmol/L and 100 μmol/L of cisplatin, at 43 °C with 50 μmol/L, 100 μmol/L, and 200 μmol/L cisplatin, at 44 °C with 50 μmol/L , 100 μmol/L, and 200 μmol/L of cisplatin), additive (at 39 °C with 100 μmol/L of cisplatin, at 41 °C with 100 μmol/L cisplatin, and at 42 °C with 50 μmol/L and 100 μmol/L of cisplatin), and antagonistic (at 39 °C with 200 μmol/L of cisplatin, at 40 °C with 200 μmol/L cisplatin, at 41 °C with 50 μmol/L and 200 μmol/L cisplatin, and at 42 °C with 200 μmol/L of cisplatin). Combined treatment of T3M4 at 41 °C with 200 μmol/L cisplatin, at 42 °C with 50 μmol/L cisplatin, and at 44 °C with 100 μmol/L and 200 μmol/L of cisplatin had an additive effect. The remaining combinations were antagonistic (Figure 5).
Annexin V-PE flow cytometry analysis was performed to evaluate rates of early apoptosis. Cisplatin induced early apoptosis in Caco-2 and T3M4 cells 1.5-fold and 3.4-fold, respectively. The number of dead/late apoptotic cells was insignificant in the following groups: 0.5% of Caco-2 cells and 2.9% of T3M4 cells. Hyperthermia of 43 °C in addition to cisplatin induced early apoptosis as compared to cells treated in normothermia by 20% in Caco-2 (1% of dead cells) and 19% (3.9% dead cells) in T3M4, respectively. Interestingly, early apoptosis was not significantly induced by isolated cisplatin treatment in AGS cells. Rates of AGS early apoptosis were not significantly induced by an isolated cisplatin treatment. However, application of combined treatment with hyperthermia and cisplatin increased early apoptosis by 61% (0.4% dead cells) (Figure 6).
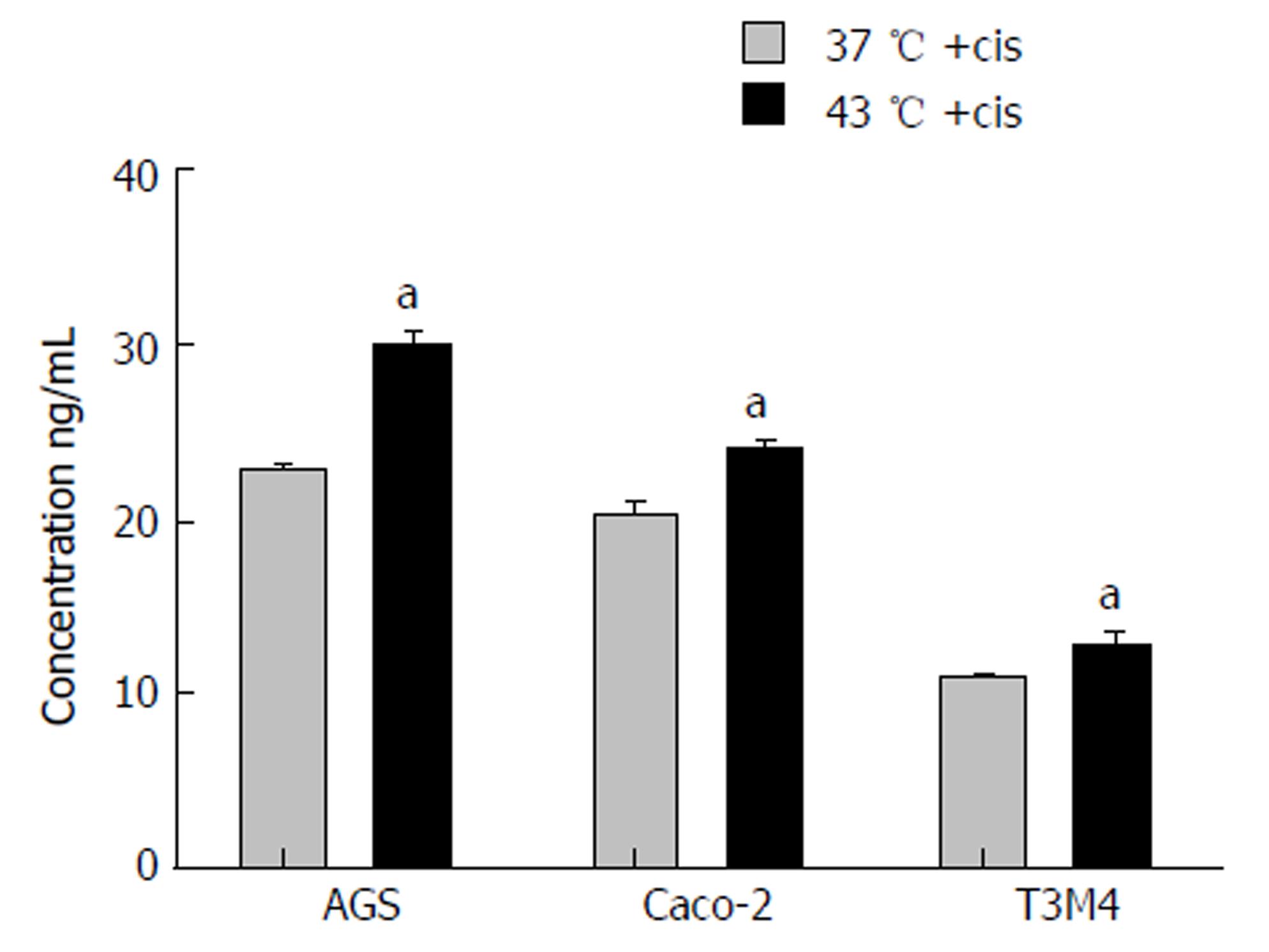
Hypothesizing that hyperthermia promotes delivery of cisplatin to cancer cells, we aimed to measure intracellular cisplatin concentrations. Analysis was performed immediately following a one-hour exposure to the IC50 of cisplatin at 37 °C and 43 °C. Overall, 43 °C promoted an increase of intracellular cisplatin concentration. The concentration was significantly increased in AGS, Caco-2, and T3M4 cells by 30%, 20%, and 18%, respectively (Figure 7).
Hyperthermal intraperitoneal chemotherapy has been applied to treat peritoneal carcinomatosis for gastric[17-19], colorectal[20], ovarian[21], and peritoneal mesothelioma[22] and has aided in prolonged long-term survival in selected patients[23-26]. In HIPEC, intraperitoneal free cancer cells and the serous surface of the bowel and peritoneum are exposed to high concentrations of chemotherapy agents. Hyperthermia itself may affect cell cytoskeletons, cell membranes, synthesis of macromolecules, and DNA repair mechanisms[9].
As the origin, genotypes, and phenotypes of free cancer cells may be quite different, it seems unreasonable to treat all peritoneal metastases the same way. Until now, basic information regarding the contribution of hyperthermia to intraperitoneal chemotherapeutic agents in different gastrointestinal cancers is poor and controversial. In this in vitro study, we have applied hyperthermia and chemotherapy for 1 h in order to mimic clinical conditions. We have performed our aimed readouts at 48 h following the experiment as the earlier readouts have not proven any changes.
We have chosen three most common peritoneum invading GI cancers in our study, selecting one cancer cell line per cancer type. It is known that various cancer cells of same cancer type are different in the manner of apoptosis, biomarker expression etc [27]. This study is an overview, which leads to a better knowledge of gastric, pancreatic and colorectal cancer cell response to simultaneous hyperthermia and cisplatin treatment.
Until now, our analyzed cells are well examined in various aspects. AGS cells in 516 analyzed genes, have 40 mutations, like KRAS, WNK1 and WNK2[28]. Overexpression of HER-2, EGFR, VEGF, Bcl-2 biomarkers was investigated in gastric cancer cells[29]. Mutations of SMAD4 and TP53 genes and overexpression of ILF3, TIMP3 genes are present in Caco-2 cells[30-32] . T3M4 are the least examined cells. No known gene mutations were found[33] and they are known to overexpress growth factors as FGFR4[34].
Our experiments revealed the differences of cell viability in response to isolated hyperthermia. We even observed positive effects of isolated hyperthermia on the viability of pancreatic cancer cells. We hypothesize that this effect could be related to the activation of the cytoprotective heat shock proteins (HSP), an effect described in other studies[35]. It is known that higher temperatures can enhance thermotolerance by activating HSPs[36]. However, gastric cancer cells (AGS) and colon cancer cells (Caco-2) responded to hyperthermia with a significant drop of viability at 43 °C. One study demonstrated that hyperthermia alone can significantly reduce viability of the Colon 26 cancer cells[37]. The investigation of the effect of local heat used for ablation of tumor nodules showed that exposing cultured Caco-2 cells to 48 °C for 2 h resulted in an approximately 80% reduction of cell viability[38]. However, in our study, the viability of Caco-2 cells decreased only by 14% after exposure to 43 °C for 1 h.
Hyperthermia may enhance the effect of chemotherapy, especially cisplatin-based treatments[6-8]. Ferraro et al[39] studied the effects of hyperthermia and cisplatin on model protein hen eggs and revealed that increased temperature enhanced cisplatin cytotoxicity.
Tang et al[13] have shown a synergistic effect of hyperthermia and chemotherapy inhibiting proliferation in six gastric cancer cell lines, including AGS, in a certain range of chemoagent (cisplatin) concentration. Moreover, hyperthermia, in addition to chemotherapy, induced cell apoptosis as the major type of death. MicroRNA-218 upregulation and increased chemosensitivity were observed in gastric cancer cells after exposing them to hyperthermal conditions[40]. However, in our study, only temperatures higher than 43 °C in addition to cisplatin exposure inhibited cell growth. In the isobologram analysis, the combination of temperature and cisplatin was antagonistic for AGS cells. On the contrary, AGS cells showed an increased viability of 33% at 42 °C. This is an important observation as it may highlight the possibility that technically incorrect temperature regimens may activate metabolism of cancer cells and increase their resistance to chemotherapy. Interestingly, we observed that application of cisplatin to gastric cancer (AGS) cells for one h had no significant impact on cell apoptosis. However, the combination of cisplatin and hyperthermia (43 °C) increased the affect by 61%. Our results suggest that temperature and cisplatin are not always acting in synergy as was shown by isobolograms.
Previous in vitro investigations have revealed that the response of the colon cancer cells to hyperthermia and chemotherapeutic drug differ by cell type. Some authors have shown that hyperthermia alone significantly decreased the viability of the Colon 26 cancer cells[37], and the cytotoxic effect of cisplatin[41] was enhanced at hyperthermal conditions. After exposure to 43 °C, the activity of oxaliplatin markedly and rapidly increased, indicating its inhibiting potential in Colon 26 cells[42]. Significant synergy of hyperthermia and chemotherapy was detected in CX-1 cells[43] in similar experimental conditions (42 °C for 1 h). Some authors described similar results in HCT116+ch2 (MMR-) cells that were resistant to heat treatment at temperatures of 41 °C and 42 °C[12]. The exposition of the colon carcinoma cell lines CX-1 and HTC 116 to 42 °C for 1 h revealed no change in cell viability[13,43]. Moreover, HCT116+ch2 (MMR-) cells exposed to a mild heat were 1.42-fold more resistant to cisplatin[12]. We observed that the addition of hyperthermia to cisplatin treatment had a slight positive influence on Caco-2 cell viability. At 43 °C, cisplatin had an insignificant effect regarding apoptosis, whereas intracellular cisplatin concentration was elevated by 26% as compared to the control. Isobologram analysis showed approximately 50% of selected cisplatin and temperature treatment combinations acted in a synergistic manner, indicating that some of hyperthermic chemotherapy regimens might be useful.
Recent studies reported that hyperthermia increased sensitivity to gemcitabine[44] and enhanced gemcitabine-related apoptotic cell death in pancreatic cancer cells[45]. Moreover, the improved drug delivery and antitumor effects in combination with mild hyperthermia were reported [46].
The combined cytotoxic effect of cisplatin and hyperthermia in T3M4 cells was evident only when the temperature was higher than 43 °C. These cells were the most sensitive to cisplatin with the lowest IC50. Cisplatin amplified apoptosis by 3.4-fold and hyperthermia increased the effect by 19%; however, it was 3-fold less in the gastric cancer cell line. Similar to AGS cells, T3M4 isobologram analysis revealed that the combination of treatments acted in an antagonistic manner in most of the selected combinations; however, in few of them, the additive effect of the two agents was revealed. An extremely limited increase of intracellular cisplatin concentration in hyperthermia could be observed in pancreatic cancer cells. Our experiments elucidated that the response of T3M4 cells differs from other studied gastrointestinal cancer cells (AGS and Caco-2). A review by Loggie et al[47] postulated that hyperthermia combined with chemotherapy is probably beneficial for less aggressive tumors and should be the standard of care for appendiceal and colorectal cancers.
Caco-2 cell results varied from the other cells in our study. In some cases, they acted in unpredictable manner. One of the possible explanations for this discrepancy, would be that Caco-2 cells can differentiate their phenotype prior to post-confluence stage, and lead to the change of morphology, degree of differentiation etc[48].
In summary, we have proven that the role of hyperthermia in addition to cisplatin is different in gastric, colonic, and pancreatic cancer cells. The overview of the basic results is shown in Table 1. Different cancer cells respond to combined treatments in different manners, and temperature-induced apoptosis is initiated by various pathways[49].
| Cell line | Effect of temperature at 43 °C on cancer cells viability | IC50 dose of cisplatin | Combined effect of temperature, at 43 °C and IC50 dose of cisplatin on cancer cells viability | Effect of temperature in addition to IC50 dose of cisplatin on cancer cell apoptosis | Effect of temperature on intracellular cisplatin concentration |
| AGS | ↓↓ | 182 | ↓ | ↑ | ↑↑↑ |
| Caco-2 | ↓ | 194 | ↓↓ | - | ↑↑ |
| T3M4 | - | 48 | - | ↑ | ↑ |
In conclusion, hyperthermia up to 43 °C in addition to cisplatin does not influence AGS, Caco-2, and T3M4 cell viability in a synergistic manner. However, some regimens of hyperthermia and cisplatin treatment are beneficial regarding the apoptotic response and an increase of intracellular cisplatin concentration.
Hyperthermal intraperitoneal chemotherapy is an option to treat peritoneum invading gastrointestinal cancer. Until now the results of hyperthermal intraperitoneal chemotherapy (HIPEC) treatment are controversy, needing unification in selected parameters of the treatment. In different cancer centers, the procedure varies in time setting, hyperthermia level and different chemotherapy drugs are used.
As HIPEC is widely used in clinical practice, still there is a lack of studies, analyzing the impact of hyperthermia and cisplatin to cancer cells. The cellular response to the treatment is still not clear. There is no clear data defining optimal timing and temperature of the procedure.
Our objective was to analyze gastric, pancreatic and colorectal cancer cells response to hyperthermia and cisplatin treatment regarding viability, change of intracellular cisplatin concentration and apoptosis rate.
We used AGS (gastric cancer), T3M4 (pancreatic cancer) and Caco-2 (colorectal cancer) cells. Mimicking HIPEC procedure, cells were treated with specific to each cell line IC50 dose of cisplatin at the temperature regimens ranging from 37 °C to 45 °C. Treatment lasted for one hour. Later cells were harvested in normothermia, changing cisplatin containing media to fresh one. Immediately after experiment, intracellular cisplatin concentration was measured, using mass spectrometer analysis. For other readouts cells were harvested for 48 hours in normal conditions. MTT test was performed for cellular viability evaluation and isobologram analysis. We used flow cytometry to determine apoptosis change of hyperthermia and cisplatin treatment.
Cells responded to hyperthermia (ranging from 38 °C to 45 °C) in a different manner. Viability of AGS cells was the most hyperthermia-dependent, decreasing by 30% (from 41 °C to 45 °C). Caco-2 cell viability had no change in the interval from 38 °C to 42 °C. Higher temperature regimens dropped its viability rate by 14%-20%. T3M4 cells reacted differently. Viability dropped until 42 °C, but at higher temperature regimens, we observed increase of viability. While in simultaneous hyperthermia and cisplatin treatment, we observed no change of viability until 41 °C in all cancer cells. Higher temperatures inhibited cell growth. Interestingly, we observed peaks of viability in AGS (42 °C, increase by 33%) and T3M4 (43 °C, increase by 32%) cells. Putting all MTT data to isobologram analysis, we observed synergistic, antagonistic, or additive effects of combined treatment. Hyperthermia and cisplatin treatment was strongly antagonistic in AGS cells. In Caco-2 cells we observed synergistic, additive and antagonistic effects of simultaneous treatment. Few combined treatment regimens were additive for T3M4 cells, and remaining antagonistic. Cisplatin induced early apoptosis in Caco-2 and T3M4 cells 1.5-fold and 3.4-fold, respectively. Hyperthermia of 43 °C in addition to cisplatin induced early apoptosis as compared to cells treated in normothermia by 20% in Caco-2 and 19% in T3M4, respectively. Hyperthermia strongly decreased intracellular cisplatin concentration in AGS, Caco-2, and T3M4 cells by 30%, 20%, and 18%, respectively.
Our data suggest that HIPEC conditions have to be cancer type dependent and well revised. Particular temperature regimens can do more harm, than benefit, by activating cell division and growth.
To get better knowledge of hyperthermia and cisplatin treatment effects, future studies should include more cancer cell lines per cancer type. Also, in vivo vehicle should be established.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lithuania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caboclo JL, Zhu YL S- Editor:Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 316] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Bloemendaal AL, Verwaal VJ, van Ruth S, Boot H, Zoetmulder FA. Conventional surgery and systemic chemotherapy for peritoneal carcinomatosis of colorectal origin: a prospective study. Eur J Surg Oncol. 2005;31:1145-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Chua TC, Esquivel J, Pelz JO, Morris DL. Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol. 2013;107:566-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Bergs JW, Franken NA, Haveman J, Geijsen ED, Crezee J, van Bree C. Hyperthermia, cisplatin and radiation trimodality treatment: a promising cancer treatment? A review from preclinical studies to clinical application. Int J Hyperthermia. 2007;23:329-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Alvarez-Berríos MP, Castillo A, Mendéz J, Soto O, Rinaldi C, Torres-Lugo M. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int J Nanomedicine. 2013;8:1003-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Gabano E, Colangelo D, Ghezzi AR, Osella D. The influence of temperature on antiproliferative effects, cellular uptake and DNA platination of the clinically employed Pt(II)-drugs. J Inorg Biochem. 2008;102:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Ried M, Potzger T, Braune N, Diez C, Neu R, Sziklavari Z, Schalke B, Hofmann HS. Local and systemic exposure of cisplatin during hyperthermic intrathoracic chemotherapy perfusion after pleurectomy and decortication for treatment of pleural malignancies. J Surg Oncol. 2013;107:735-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Schaaf L, Schwab M, Ulmer C, Heine S, Mürdter TE, Schmid JO, Sauer G, Aulitzky WE, van der Kuip H. Hyperthermia Synergizes with Chemotherapy by Inhibiting PARP1-Dependent DNA Replication Arrest. Cancer Res. 2016;76:2868-2875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Sottile ML, Losinno AD, Fanelli MA, Cuello-Carrión FD, Montt-Guevara MM, Vargas-Roig LM, Nadin SB. Hyperthermia effects on Hsp27 and Hsp72 associations with mismatch repair (MMR) proteins and cisplatin toxicity in MMR-deficient/proficient colon cancer cell lines. Int J Hyperthermia. 2015;31:464-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Tang R, Zhu ZG, Qu Y, Li JF, Ji YB, Cai Q, Liu BY, Yan M, Yin HR, Lin YZ. The impact of hyperthermic chemotherapy on human gastric cancer cell lines: preliminary results. Oncol Rep. 2006;16:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, Arnold S, Fraker DL, Alexander HR. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol. 1997;4:141-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunol Immunother. 2006;55:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3327] [Cited by in F6Publishing: 3648] [Article Influence: 202.7] [Reference Citation Analysis (0)] |
| 17. | Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol. 2016;22:1114-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 67] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Yonemura Y, Canbay E, Li Y, Coccolini F, Glehen O, Sugarbaker PH, Morris D, Moran B, Gonzaletz-Moreno S, Deraco M. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42:1123-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Yuan M, Wang Z, Hu G, Yang Y, Lv W, Lu F, Zhong H. A retrospective analysis of hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Mol Clin Oncol. 2016;5:395-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Simkens GA, van Oudheusden TR, Braam HJ, Wiezer MJ, Nienhuijs SW, Rutten HJ, van Ramshorst B, de Hingh IH. Cytoreductive surgery and HIPEC offers similar outcomes in patients with rectal peritoneal metastases compared to colon cancer patients: a matched case control study. J Surg Oncol. 2016;113:548-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22:1570-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 22. | Kepenekian V, Elias D, Passot G, Mery E, Goere D, Delroeux D, Quenet F, Ferron G, Pezet D, Guilloit JM, Meeus P, Pocard M, Bereder JM, Abboud K, Arvieux C, Brigand C, Marchal F, Classe JM, Lorimier G, De Chaisemartin C, Guyon F, Mariani P, Ortega-Deballon P, Isaac S, Maurice C, Gilly FN, Glehen O; French Network for Rare Peritoneal Malignancies (RENAPE). Diffuse malignant peritoneal mesothelioma: Evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE Database: Multi-Institutional Retrospective Study. Eur J Cancer. 2016;65:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Michalakis J, Georgatos SD, de Bree E, Polioudaki H, Romanos J, Georgoulias V, Tsiftsis DD, Theodoropoulos PA. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann Surg Oncol. 2007;14:1220-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Zhang XL, Hu AB, Cui SZ, Wei HB. Thermotherapy enhances oxaliplatin-induced cytotoxicity in human colon carcinoma cells. World J Gastroenterol. 2012;18:646-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Steel GG, Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979;5:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 592] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1396] [Cited by in F6Publishing: 1415] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 27. | Li Q, Wennborg A, Aurell E, Dekel E, Zou JZ, Xu Y, Huang S, Ernberg I. Dynamics inside the cancer cell attractor reveal cell heterogeneity, limits of stability, and escape. Proc Natl Acad Sci USA. 2016;113:2672-2677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Zang ZJ, Ong CK, Cutcutache I, Yu W, Zhang SL, Huang D, Ler LD, Dykema K, Gan A, Tao J. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Cancer Res. 2011;71:29-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Jin Z, Jiang W, Wang L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis (Review). Oncol Lett. 2015;9:1502-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C, Lipton L. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013;73:725-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 31. | Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 32. | Tremblay E, Auclair J, Delvin E, Levy E, Ménard D, Pshezhetsky AV, Rivard N, Seidman EG, Sinnett D, Vachon PH. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J Cell Biochem. 2006;99:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Klöppel G, Kalthoff H. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Shah RN, Ibbitt JC, Alitalo K, Hurst HC. FGFR4 overexpression in pancreatic cancer is mediated by an intronic enhancer activated by HNF1alpha. Oncogene. 2002;21:8251-8261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Guo Y, Ziesch A, Hocke S, Kampmann E, Ochs S, De Toni EN, Göke B, Gallmeier E. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. J Cell Mol Med. 2015;19:340-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Liu T, Ye YW, Zhu AL, Yang Z, Fu Y, Wei CQ, Liu Q, Zhao CL, Wang GJ, Zhang XF. Hyperthermia combined with 5-fluorouracil promoted apoptosis and enhanced thermotolerance in human gastric cancer cell line SGC-7901. Onco Targets Ther. 2015;8:1265-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Kudo M, Asao T, Hashimoto S, Kuwano H. Closed continuous hyperthermic peritoneal perfusion model in mice with peritoneal dissemination of colon 26. Int J Hyperthermia. 2004;20:441-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Kirstein MN, Root SA, Moore MM, Wieman KM, Williams BW, Jacobson PA, Marker PH, Tuttle TM. Exposure-response relationships for oxaliplatin-treated colon cancer cells. Anticancer Drugs. 2008;19:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Ferraro G, Pica A, Russo Krauss I, Pane F, Amoresano A, Merlino A. Effect of temperature on the interaction of cisplatin with the model protein hen egg white lysozyme. J Biol Inorg Chem. 2016;21:433-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014;20:11347-11355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res. 2011;4:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Okayama T, Kokura S, Ishikawa T, Adachi S, Hattori T, Takagi T, Handa O, Naito Y, Yoshikawa T. Antitumor effect of pretreatment for colon cancer cells with hyperthermia plus geranylgeranylacetone in experimental metastasis models and a subcutaneous tumor model of colon cancer in mice. Int J Hyperthermia. 2009;25:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Yoo J, Lee YJ. Effect of hyperthermia and chemotherapeutic agents on TRAIL-induced cell death in human colon cancer cells. J Cell Biochem. 2008;103:98-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Vertrees RA, Das GC, Popov VL, Coscio AM, Goodwin TJ, Logrono R, Zwischenberger JB, Boor PJ. Synergistic interaction of hyperthermia and Gemcitabine in lung cancer. Cancer Biol Ther. 2005;4:1144-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, Naito Y, Yoshikawa T. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperthermia. 2009;25:210-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Kirui DK, Celia C, Molinaro R, Bansal SS, Cosco D, Fresta M, Shen H, Ferrari M. Mild hyperthermia enhances transport of liposomal gemcitabine and improves in vivo therapeutic response. Adv Healthc Mater. 2015;4:1092-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Loggie BW, Thomas P. Gastrointestinal Cancers With Peritoneal Carcinomatosis: Surgery and Hyperthermic Intraperitoneal Chemotherapy. Oncology (Williston Park). 2015;29:515-521. [PubMed] [Cited in This Article: ] |
| 48. | Ferraretto A, Gravaghi C, Donetti E, Cosentino S, Donida BM, Bedoni M, Lombardi G, Fiorilli A, Tettamanti G. New methodological approach to induce a differentiation phenotype in Caco-2 cells prior to post-confluence stage. Anticancer Res. 2007;27:3919-3925. [PubMed] [Cited in This Article: ] |
| 49. | Ahmed K, Tabuchi Y, Kondo T. Hyperthermia: an effective strategy to induce apoptosis in cancer cells. Apoptosis. 2015;20:1411-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |