Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3146
Revised: April 2, 2004
Accepted: April 9, 2004
Published online: November 1, 2004
AIM: To assess the possibility of non-invasive screening of atrophic chronic gastritis for preventing further development of gastric cancer.
METHODS: One hundred and seventy-eight consecutive Helicobacter pylori (H pylori)-positive dyspeptic patients after detection of serum levels of pepsinogen-1 (PG-1) and gastrin-17 (G-17) by enzyme immunoassay were proposed for endoscopy and histology. The serologic and morphologic results were compared with estimating the sensitivity, specificity and prognostic values of the tests.
RESULTS: There was statistically significant reverse dependence between the grade of stomach mucosal antral or corpus atrophy and the proper decreasing of serum G17 or PG1 levels. The serologic method was quite sensitive in the diagnosis of non-atrophic and severe antral and corpus gastritis. Also, it was characterized by the high positive and negative prognostic values.
CONCLUSION: Detection of serum G-17 and PG1 levels can be offered as the screening tool for atrophic gastritis. The positive serologic results require further chromoendoscopy with mucosal biopsy, for revealing probable progressing of atrophic process with development of intestinal metaplasia, dysplasia or gastric cancer.
- Citation: Pasechnikov VD, Chukov SZ, Kotelevets SM, Mostovov AN, Mernova VP, Polyakova MB. Possibility of non-invasive diagnosis of gastric mucosal precancerous changes. World J Gastroenterol 2004; 10(21): 3146-3150
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3146
According to the modern representations about stages of gastric carcinogenesis, it is supposed that chronic Helicobacter pylori (H pylori) infection is the trigger mechanism in 60%-90% of gastric cancer cases[1,2]. In 1975, Correa proposed the consecutive line of events leading to gastric cancer development: Normal mucosa - chronic active gastritis - chronic atrophic gastritis - intestinal metaplasia - dysplasia - carcinoma in situ. At present, this cascade of changes associates with a trigger role of H pylori and is called “Correa’s gastric precancerous cascade”[2,3]. The early detection of gastric precancerous changes, mainly atrophic gastritis, may be helpful in prevention of gastric cancer or in diagnosis of cancer at curable stages. So, there is a critical need for valid diagnostic methods of stomach mucosal atrophy, that would be inexpensive and non-invasive, that is, suitable for screening of large groups of people.
Taking into account the known interrelations between the morphological status and functional activity of gastric corpus and antral mucosa and the secretion of, respectively, pepsinogen 1 and gastrin 17[4,5], we carried out a prospective study with the aim to detect H pylori-induced gastric precancerous changes, that were leading to cancer formation, and to evaluate the possibility of non-invasive screening of dyspeptic patients.
The study was carried out in a group of dyspeptic H pylori-infected patients.
H pylori antibodies (Hp-Ab), serum levels of pepsinogen I (PGI) and gastrin-17 (G-17) were analyzed by enzyme immunoassay with Biohit GastroPanel® (Biohit Plc, Helsinki, Finland). According to the instructions of its manufacturer, serum levels of PG1 < 25 μg/L were estimated as markers of gastric corpus atrophy, serum levels of G17 < 5 pmol/L were estimated as markers of gastric antral atrophy, serum levels of G17 < 10 pmol/L in a combination with serum levels of PG1 < 50 μg/L were estimated as markers of mild gastric corpus atrophy. HPAb IgG titers were estimated as follows: < 32 enzyme immunoassay unit (EIU)- negative result, 32 - 44 EIU - doubtful result, > 44 EIU - positive result. The numerical meanings of researched parameters were analyzed by the program GastroSoft® (Biohit Plc, Helsinki, Finland) enclosed to test-system Biohit GastroPanel®. On the basis of inserted data, the program composed the diagnosis in a view of the presence or absence of H pylori-infection and mucosal atrophy, with the estimation of gastric cancer or peptic ulcer risk and with recommendations on the treatment according to Maastricht-2 consensus.
After getting the GastroSoft® diagnosis, we randomized 178 patients for the following study. The patients underwent the upper gastrointestinal endoscopy with subsequent biopsy of the antral and corpus mucosa. To increase the accuracy of endoscopic diagnosis, we carried out an additional chromoendoscopy with methylene blue staining allowing the detection of foci of intestinal metaplasia (IM) of gastric mucosa which were unrecognized by routine endoscopy. Biopsy specimens were stained with hematoxylin-eosin and PAS reaction in combination with alcian blue at pH2.5. The grade of stomach mucosal atrophy was estimated from 0 to 3 according to Houston visual analogous scale.
Statistical analysis was used to calculate the statistical significance of received data (Mann-Whitney criterion). Spearman’s correlation coefficient (rs), positive predictive value (PPV) and negative predictive value (NPV) of diagnosis by Biohit GastroPanel®.
Of the 178 patients, non-atrophic chronic gastritis (no antral atrophy and no corpus atrophy) was detected only in 5 patients. The mean of serum PG-1, G-17 and anti-H pylori IgG in this group was 85.28 ± 35.07 μg/L, 14.44 ± 1.90 pmol/L and 85.68 ± 17.81 EIU, respectively. In all these cases there was no IM or dysplasia in stomach mucosal epithelium.
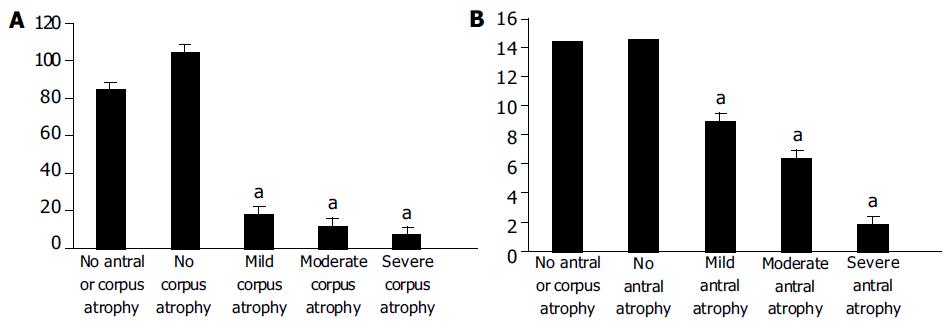
The morphological status of gastric corpus mucosa was compared with serum PG-1 levels (Figure 1A). The non-atrophic corpus mucosa was detected in 99 (55.62%) of 178 patients, mild atrophy of corpus mucosa was detected in 17 (9.55%) patients, moderate atrophy of corpus mucosa was detected in 36 (20.22%) patients, severe atrophy of corpus mucosa was detected in 26 (14.61%) patients.
The statistical analysis revealed that there were marked differences between the levels of PG1 in non-atrophic and atrophic corpus gastritis. The levels of PG1 in mild, moderate and severe corpus atrophy were significantly lower (P < 0.0001) than those in non-atrophic state. In turn, the levels of PG1 in mild, moderate and severe corpus atrophy were significantly different from each other (P < 0.0001).
The morphological status of gastric antral mucosa was compared with serum G-17 levels (Figure 1B). The non-atrophic antral mucosa was detected in 12 (6.74%) of 178 patients. Mild atrophy of antral mucosa was detected in 31 (17.42%) patients. Moderate antral mucosa atropy was detected in 69 (38.76%) patients. Severe antral mucosal atrophy was detected in 66 (37.08%) patients.
The statistical analysis revealed that there were marked differences between the levels of G17 in non-atrophic and atrophic antral gastritis. The latter was significantly lower (P < 0.0001). Moreover, the levels of G17 in mild, moderate and severe antral atrophy were significantly different from each other (P < 0.0001).
Both antral and corpus stomach mucosal atrophy were not accompanied with statistically significant changes in anti-H pylori IgG serum titers (Table 1).
| Anti-H pylori IgG, EIU | |
| No antral or corpus atrophy | 85.68 ± 17.81 |
| No antral atrophy | 62.29 ± 12.02 |
| Mild antral atrophy | 78.25 ± 5.86 |
| Moderate antral atrophy | 74.08 ± 4.39 |
| Severe antral atrophy | 73.38 ± 5.38 |
| No corpus atrophy | 68.76 ± 8.35 |
| Mild corpus atrophy | 77.21 ± 6.13 |
| Moderate corpus atrophy | 76.47 ± 5.04 |
| Severe corpus atrophy | 72.61 ± 4.72 |
We detected intestinal metaplasia in 119 of 178 patients. The comparison of the grade of IM and the serum levels of PG-1 and G-17 showed the absence of invariables and statistically significant dependence between these parameters, except for the cases of moderate IM (Table 2).
Dysplasia of stomach mucosal epithelium was detected in 113 patients. We revealed the statistically significant dependence between the grade of dysplasia and the increased serum levels of PG-1, and the decreased levels of G-17 (Table 3), except the cases of severe dysplasia where the number of patients was not quite sufficient (6 subjects).
Among the 178 consecutive patients we detected 6 cases of gastric cancer: 2 early cancers and 4 progressed tumors. The mean of serum PG-1, G-17 and anti-H pylori IgG in these cases was 46.73 ± 22.25 μg/L, 4.78 ± 2.086 pmol/L and 89.5 ± 11.7 EIU, respectively.
In general, our results showed that there was a statistically significant dependence between the presence and severity of stomach mucosal atrophy (antral or corpus) and the proper serologic markers (G17 or PG1) in H pylori-associated chronic gastritis. On the other hand, the presence and degree of IM did not correspond to the serum level of G17 or PG1. Thus, the serologic screening by means of Biohit GastroPanel® was useful for the selection of patients with stomach mucosal atrophy by subsequent thorough endoscopical and histological examination for the possible development of precancerous or malignant changes in stomach mucosa.
Furthermore, we compared the results of the serology, endoscopy and histology by Spearman’s correlation coefficient. As shown in Table 4, the correlation between the results of routine endoscopy and histology was positive, but obviously weaker than the correlation between the results of chromoendoscopy and histology. As it was expected, there was a strong reverse correlation between the presence and degree of stomach mucosal atrophy and the serum levels of proper markers of its functional activity. The correlation between the degree of stomach mucosal atrophy and the subsequent morphological changes of mucosa - IM and dysplasia, was positive but quite weak.
| Parameters | rs |
| Detection of IM by endoscopy and histology | 0.41 |
| Detection of IM by chromoendoscopy and histology | 0.92 |
| Detection of antral atrophy by histology | -0.75 |
| and serum levels of G-17 | |
| Detection of corpus atrophy by histology | -0.71 |
| and serum levels of PG-1 | |
| Degree of stomach mucosal atrophy and IM | 0.21 |
| Degree of stomach mucosal atrophy and dysplasia | 0.23 |
| Degree of IM and dysplasia | 0.41 |
The levels of sensitivity and specificity, PPV and NPV of Biohit GastroPanel® in diagnosis of stomach mucosal atrophy are presented in Tables 5 and 6.
| Degree of antral atrophy | Se (%) | Sp (%) | PPV (%) | NPV (%) |
| (by histology) | ||||
| No | 83 | 95 | 53 | 99 |
| Mild | 61 | 84 | 45 | 91 |
| Moderate | 67 | 90 | 81 | 81 |
| Severe | 89 | 99 | 98 | 94 |
| Degree of corpus atrophy | Se (%) | Sp (%) | PPV (%) | NPV (%) |
| (by histology) | ||||
| No | 92 | 97 | 98 | 91 |
| Mild | 71 | 92 | 48 | 97 |
| Moderate | 72 | 96 | 81 | 93 |
| Severe | 88 | 97 | 82 | 98 |
Thus, the investigated non-invasive method was quite sensitive in the diagnosis of non-atrophic and severe antral and corpus gastritis. Also, this method was characterized by the high PPV and NPV (except the cases of mild stomach mucosal atrophy).
Finally, we carried out the comparison of sensitivity and specificity, PPV and NPV for routine endoscopy and chromoendoscopy in the diagnosis of IM (Table 7).
| Routine endoscopy | Chromoendoscopy | |||||||
| Se (%) | Sp (%) | PPV (%) | NPV (%) | Se (%) | Sp (%) | PPV (%) | NPV (%) | |
| No | 98 | 6 | 35 | 86 | 94 | 99 | 98 | 97 |
| Mild | 4 | 98 | 50 | 68 | 88 | 88 | 79 | 94 |
| Moderate | no data | no data | no data | no data | 71 | 95 | 80 | 92 |
| Severe | 6 | 99 | 33 | 89 | 82 | 98 | 82 | 98 |
As a result, we established the obvious advantage of a method of chromoendoscopy in the diagnosis of IM, that had doubtless diagnostic importance in revealing precancerous changes of gastric mucosa.
Chronic H pylori gastritis eventually could lead in more than half of the affected subjects to a gradual loss of glandular structures with its specialized cells and a collapse of the reticulin skeleton of the mucosa, a condition of atrophic gastritis[8]. As a result, the glandular layer of the mucosa became thinner, and glands were replaced by fibrosis and intestinal metaplasia. The major clinical importance of this condition was that it could significantly increase the risk for the intestinal type of gastric cancer. This risk might be elevated up to 90 - fold in subjects with severe atrophic gastritis throughout the complete stomach[9]. The annual incidence of gastric cancer among patients with atrophic gastritis varied in cohort studies between 0.3% and 1.0%[10]. This could explain the interest in the diagnosis of atrophic gastritis. At present, there is a wide circle of questions related to the diagnosis of critical stages of gastric carcinogenesis - gastric epithelial atrophy, intestinal metaplasia and dysplasia. Therefore, it is extremely important to recognize dyspeptic patients who have very high risk of gastric malignant changes and require dynamic surveillance with the purpose of early revealing of the preneoplastic changes in stomach mucosa. Atrophic gastritis is a serious disease, which often does not receive much attention. The relationship between gastritis, atrophic gastritis and other diseases of the stomach is based on the fact that infection and atrophy could alter the physiological functions of the stomach[11] and influence the growth and growth control of epithelial cells in the stomach. These consequences varied depending on whether the changes of the gastric mucosa caused by gastritis were located in the antrum or the corpus or both.
The most accurate diagnostic method of gastrointestinal tract diseases is endoscopy with subsequent biopsy, which should be made in all patients with the presence of clinical symptoms. However, because of patchy characteristics of atrophic changes in stomach mucosa, some histological researches could give false - negative results. Besides, biopsy was an expensive and labor-consuming method of research[12], so it could not be carried out for all patients in succession. Contrarily, due to invasiveness of biopsy, it is expedient to make only for monitoring precancerous changes in stomach mucosa. For the selection of patients recommended to biopsy, the presence of a screening method is necessary. Such a method should be capable of reflecting objectively the functional condition of stomach mucosa and its morphological status.
It has been known for over two decades that atrophic gastritis of the corpus and fundus of the stomach can be determined reliably by measuring the serum level of pepsinogen I (PGI) or the PGI/PGII ratio from a blood sample[13-15]. However, it has not been possible to determine from a blood sample the types of atrophic gastritis in which the atrophic changes are located solely in the antrum. The GastroPanel® serum test also enables the determination of atrophic gastritis of this antrum-limited subtype.
Group I pepsinogens are synthesized solely in the oxyntic glands and mucous neck cells of the gastric corpus. On the other hand, however, pepsinogens of group II are uniformly formed in the glands of the entire stomach and to some extent also in the Brunner glands in the first part of the duodenum.
The majority of pepsinogens are secreted into the lumen of the stomach where they are metabolized into an active pepsin. A small proportion of the pepsinogens leak for one reason or another into the blood circulation. In case of atrophic corpus gastritis, the level of serum pepsinogen I decreases whereas the level of pepsinogen II remains stable or decreases slightly. The level of serum pepsinogen I or the ratio of serum pepsinogen I to pepsinogen II could reflect with high reliability the number of cells and oxyntic glands in the corpus area of the stomach, i.e., they could reflect the degree of atrophy of the corpus mucosa[14-16]. As the severity of atrophic corpus gastritis corpus increases, the level of serum pepsinogen I or the PGI/PGII ratio decreases.
Gastrin is synthesized in G-cells, which are found in the gastric antrum. The gastrin secreted by the antrum is over 90% of type G-17 whereas the gastrin secreted by the duodenum is primarily type G-34. The fasting serum gastrin is primarily in the form of G-34 but the proportion of type G-17 increases after the dietary stimulus. The secretion of gastrin-17 can be studied with a simple protein stimulation test. First, a blood sample is taken after fasting, after which the patient eats a protein-rich meal. The maximum increase in the level of gastrin-17 can be seen in the serum within 20 min. If the serum gastrin does not increase as a result of protein or other physiological stimulation it is an indication of the loss of gastrin secreting G cells, i.e., an indication of the atrophy of the antrum mucosa. It is possible to make indirect conclusions of the status of the antrum mucosa by simultaneously assaying the serum gastrin and acid output[17]. In the cases with atrophic antral gastritis and loss of antral G cells, serum gastrin remains low although the stomach is achlorhydric or hypochlorhydric.
Several research groups have renewed the interest in serology for atrophic gastritis by combining gastrin and pepsinogens with H pylori serology. In this issue, Väänänen and colleagues presented a smart algorithm for the differentiation in both antrum and corpus between atrophic and non-atrophic gastritis[18]. The algorithm was tested in a cross-sectional study correlating gastric mucosal histology with H pylori IgG serum antibodies, serum PGI levels, and fasting and postprandial serum gastrin-17 levels. It appeared that in roughly 80% of the 404 cases tested, histology and serology matched a similar diagnosis. Sixty (15%) of the 404 subjects had atrophic gastritis, 6 (1%) had previously undergone antral resection, and 340 (84%) had a non-atrophic gastric mucosa either with or without inflammation. In this population with a rather low prevalence of atrophic gastritis, the negative predictive value of the serology panel was 93%-97% and the positive predictive value was 64%-75%. For these calculations, the authors combined all subjects with atrophic gastritis of the antrum, the corpus, or both. The data, however, showed that the serology panel performed much better in diagnosing atrophic gastritis in the corpus than in the antrum. Only 19 (50%) of the 38 patients diagnosed by serology as having antrum atrophic gastritis had this condition confirmed by histology. This revival of interest in the serological testing of the condition of the gastric mucosa is of importance, given the fact that H pylori eradication may cure gastritis and help to prevent further progression of gland loss. It is likely that this might also reduce the risk for gastric cancer[19], although many more data on this are needed. Screening and treatment of H pylori infection might in theory be cost-effective for the prevention of gastric cancer[12].
Sipponen et al[20] have recently shown that simultaneous detection of serum concentrations of PG1 and G17 and HPAb titers is an effective method for non-invasive screening and diagnosis of atrophic gastritis using blood samples of the patients.
In our present research, the use of the test - system GastroPanel® allowed to receive statistically significant differences between the serum concentrations of PG1 and G17 depending on a degree of stomach mucosal atrophy. We obtained the very satisfactory means of the PPV and NPV of GastroPanel® test in revealing the atrophic state of stomach mucosa. Moreover, this test was sufficiently sensitive and specific as it was proven by chromoendoscopy and histology.
Thus, our study confirmed the usefulness of the test - system GastroPanel® as a “serologic biopsy” for authentic and non-invasive diagnosis of atrophic changes of stomach mucosa in patients with dyspepsia associated with H pylori - infection. Besides, we managed to show the advantage of a chromoendoscopy method prior to routine endoscopy in the diagnosis of intestinal metaplasia. Now is the time, almost a decade after the conclusion of the World Health Organization (WHO) about H pylori as a class I carcinogen[21], to use this serology for further studies in selected and general populations. This will allow evaluation of the feasibility of screening and treatment for gastritis and prevention of gastric cancer.
In conclusion, the noninvasive detection of gastric mucosal atrophy by means of enzyme immunoassay with assessment of G-17 and PG1 levels can be offered as the screening tool for gastric precancerous conditions. On the other hand, this method does not allow to diagnose intestinal metaplasia and cancer development in stomach mucosa. Therefore, the results of serological screening indicating the stomach mucosal atrophy require carrying out the chromoendoscopy with subsequent mucosal biopsy, for revealing probable progressing of atrophic process with development of intestinal metaplasia, dysplasia or gastric cancer.
Edited by Wang XL Proofread by Xu FM
| 1. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 846] [Cited by in F6Publishing: 830] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 3. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] [Cited in This Article: ] |
| 4. | Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pyloriinfection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Sipponen P, Ranta P, Helske T, Kääriäinen I, Mäki T, Linnala A, Suovaniemi O, Alanko A, Härkönen M. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand J Gastroenterol. 2002;37:785-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2466] [Cited by in F6Publishing: 2324] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 7. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 725] [Cited by in F6Publishing: 682] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 538] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 262] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Kuipers EJ. Review article: Relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12 Suppl 1:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Varis K, Ihamäki T, Härkönen M, Samloff IM, Siurala M. Gastric morphology, function, and immunology in first-degree relatives of probands with pernicious anemia and controls. Scand J Gastroenterol. 1979;14:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Miki K, Ichinose M, Ishikawa KB, Yahagi N, Matsushima M, Kakei N, Tsukada S, Kido M, Ishihama S, Shimizu Y. Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn J Cancer Res. 1993;84:1086-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Yoshihara M, Sumii K, Haruma K, Kiyohira K, Hattori N, Kitadai Y, Komoto K, Tanaka S, Kajiyama G. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am J Gastroenterol. 1998;93:1090-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Kekki M, Samloff IM, Varis K, Ihamäki T. Serum pepsinogen I and serum gastrin in the screening of severe atrophic corpus gastritis. Scand J Gastroenterol Suppl. 1991;186:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Varis K, Sipponen P, Laxén F, Samloff IM, Huttunen JK, Taylor PR, Heinonen OP, Albanes D, Sande N, Virtamo J. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Helsinki Gastritis Study Group. Scand J Gastroenterol. 2000;35:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Sipponen P, Valle J, Varis K, Kekki M, Ihamäki T, Siurala M. Fasting levels of serum gastrin in different functional and morphologic states of the antrofundal mucosa. An analysis of 860 subjects. Scand J Gastroenterol. 1990;25:513-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Väänänen nen H, Vauhkonen M, Helske T, Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, Koskenpato J, Sotka M, Turunen M, Sandstrom R. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gas-trin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885–891. [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Chun Y, Wong B, Lam SK, Wong WM, Zheng T, Chen J, Chen B. Eradicating Helicobacter pylori infection in general population prevents gastric cancer: a 7-year prospective randomized pla-cebo-controlled study. Gastroenterology. 2002;122:A588. [Cited in This Article: ] |
| 20. | Suovaniemi O, Harkonen M, Paloheimo L, Sipponen P. GastroPanel: diagnosing atrophic gastritis from serum – pro-viding a tool for evidence-based medicine. Business Briefing: Global Health Care 2003; 1-4. [Cited in This Article: ] |
| 21. | International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 61: Schistosomes, liver flukes and Helicobacter pylori. Lyon: IARC. 1994;. [Cited in This Article: ] |