Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort
Abstract
:1. Introduction
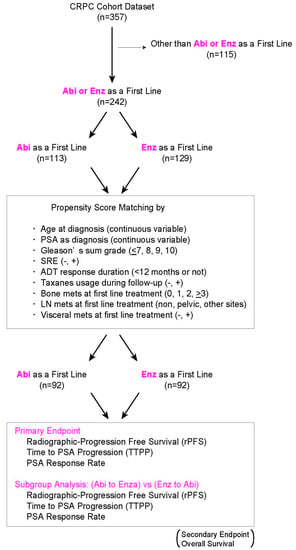
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| rPFS | radiographic progression-free survival |
| OS | overall survival |
| CRPC | castration-resistant prostate cancer |
| ASIs | androgen signaling inhibitors |
| TTPP | time to PSA progression |
| ADT | androgen deprivation therapy |
| RCT | randomized control trial |
| SCC | Spearman’s correlation coefficient |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mazzu, Y.Z.; Armenia, J.; Chakraborty, G.; Yoshikawa, Y.; Coggins, S.; Nandakumar, S.; Gerke, T.; Pomerantz, M.; Qiu, X.; Zhao, H.; et al. A novel mechanism driving poor-prognosis prostate cancer: Overexpression of the DNA repair gene, ribonucleotide reductase small subunit M2 (RRM2). Clin. Cancer Res. 2019, 25, 4480–4492. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Du, S.Y.; Armenia, J.; Qu, F.; Fan, J.; Wang, X.; Fei, T.; Komura, K.; Liu, S.X.; Lee, G.M.; et al. Expression of lncRNA MIR222HG co-transcribed from the miR-221/222 gene promoter facilitates the development of castration-resistant prostate cancer. Oncogenesis 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Yoshikawa, Y.; Shimamura, T.; Chakraborty, G.; Gerke, T.A.; Hinohara, K.; Chadalavada, K.; Jeong, S.H.; Armenia, J.; Du, S.Y.; et al. ATR inhibition controls aggressive prostate tumors deficient in Y-linked histone demethylase KDM5D. J. Clin. Invest. 2018, 128, 2979–2995. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Jeong, S.H.; Hinohara, K.; Qu, F.; Wang, X.; Hiraki, M.; Azuma, H.; Lee, G.S.; Kantoff, P.W.; Sweeney, C.J. Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc. Natl. Acad. Sci. USA 2016, 113, 6259–6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bossi, A.; Bristow, R.; Carver, B.; Castellano, D.; Chung, B.H.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. 2017, 73, 178–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiong, E.; Murphy, D.G.; Akaza, H.; Buchan, N.C.; Chung, B.H.; Kanesvaran, R.; Khochikar, M.; Letran, J.; Lojanapiwat, B.; Ng, C.F.; et al. Management of patients with advanced prostate cancer in the Asia Pacific region: ‘real-world’ consideration of results from the Advanced Prostate Cancer Consensus Conference (APCCC) 2017. BJU Int. 2019, 123, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Eigl, B.J.; Murray, R.N.; Kollmannsberger, C.; Chi, K.N. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur. Urol. 2015, 67, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Badrising, S.; van der Noort, V.; van Oort, I.M.; van den Berg, H.P.; Los, M.; Hamberg, P.; Coenen, J.L.; van den Eertwegh, A.J.; de Jong, I.J.; Kerver, E.D.; et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 2014, 120, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Brasso, K.; Thomsen, F.B.; Schrader, A.J.; Schmid, S.C.; Lorente, D.; Retz, M.; Merseburger, A.S.; von Klot, C.A.; Boegemann, M.; de Bono, J. Enzalutamide Antitumour Activity Against Metastatic Castration-resistant Prostate Cancer Previously Treated with Docetaxel and Abiraterone: A Multicentre Analysis. Eur. Urol. 2015, 68, 317–324. [Google Scholar] [CrossRef] [PubMed]
- David, T.; Natalie, C.; Omi, P. Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate resistant prostate cancer (mCRPC): Results from an expanded access program. J. Clin. Oncol. 2014, 32, 188. [Google Scholar]
- Loriot, Y.; Bianchini, D.; Ileana, E.; Sandhu, S.; Patrikidou, A.; Pezaro, C.; Albiges, L.; Attard, G.; Fizazi, K.; De Bono, J.S.; et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 2013, 24, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Maughan, B.L.; Luber, B.; Nadal, R.; Antonarakis, E.S. Comparing Sequencing of Abiraterone and Enzalutamide in Men with Metastatic Castration-Resistant Prostate Cancer: A Retrospective Study. Prostate 2017, 77, 33–40. [Google Scholar] [CrossRef]
- Mori, K.; Kimura, T.; Onuma, H.; Kimura, S.; Yamamoto, T.; Sasaki, H.; Miki, J.; Miki, K.; Egawa, S. Lactate dehydrogenase predicts combined progression-free survival after sequential therapy with abiraterone and enzalutamide for patients with castration-resistant prostate cancer. Prostate 2017, 77, 1144–1150. [Google Scholar] [CrossRef]
- Noonan, K.L.; North, S.; Bitting, R.L.; Armstrong, A.J.; Ellard, S.L.; Chi, K.N. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 2013, 24, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Schrader, A.J.; Boegemann, M.; Ohlmann, C.H.; Schnoeller, T.J.; Krabbe, L.M.; Hajili, T.; Jentzmik, F.; Stoeckle, M.; Schrader, M.; Herrmann, E.; et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 2014, 65, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Maughan, B.L.; Akamatsu, S.; Kobayashi, T.; Yamasaki, T.; Inoue, T.; Kamba, T.; Ogawa, O.; Antonarakis, E.S. Exploring the optimal sequence of abiraterone and enzalutamide in patients with chemotherapy-naive castration-resistant prostate cancer: The Kyoto-Baltimore collaboration. Int. J. Urol. 2017, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, F.B.; Roder, M.A.; Rathenborg, P.; Brasso, K.; Borre, M.; Iversen, P. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand. J. Urol. 2014, 48, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, N.; Yamada, Y.; Tabata, K.I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H.; Yano, A.; Kawakami, S.; et al. Abiraterone Followed by Enzalutamide Versus Enzalutamide Followed by Abiraterone in Chemotherapy-naive Patients with Metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Hara, T.; Tamura, K.; Sugiyama, T.; Furuse, H.; Ozono, S.; Fujisawa, M. Comparative Assessment of Efficacies Between 2 Alternative Therapeutic Sequences with Novel Androgen Receptor-Axis-Targeted Agents in Patients with Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e591–e597. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Chowdhury, S.; Feyerabend, S.; Elliott, T.; Grande, E.; Melhem-Bertrandt, A.; Baron, B.; Hirmand, M.; Werbrouck, P.; Fizazi, K. Antitumour Activity and Safety of Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Previously Treated with Abiraterone Acetate Plus Prednisone for ≥24 weeks in Europe. Eur. Urol. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Khalaf, D.; Annala, M.; Finch, D.L.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Beja, K.; Vandekerkhove, G.R.; Gleave, M.; et al. Phase 2 randomized cross-over trial of abiraterone + prednisone (ABI+P) vs. enzalutamide (ENZ) for patients (pts) with metastatic castration resistant prostate cancer (mCPRC): Results for 2nd-line therapy. J. Clin. Oncol. 2018, 36, 5015. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Beer, T.M.; Loriot, Y.; Higano, C.S.; Armstrong, A.J.; Sternberg, C.N.; de Bono, J.S.; Tombal, B.; Parli, T.; Bhattacharya, S.; et al. Radiographic Progression-Free Survival as a Clinically Meaningful End Point in Metastatic Castration-Resistant Prostate Cancer: The PREVAIL Randomized Clinical Trial. JAMA Oncol. 2018, 4, 694–701. [Google Scholar] [CrossRef]
- Morris, M.J.; Molina, A.; Small, E.J.; de Bono, J.S.; Logothetis, C.J.; Fizazi, K.; de Souza, P.; Kantoff, P.W.; Higano, C.S.; Li, J.; et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J. Clin. Oncol. 2015, 33, 1356–1363. [Google Scholar] [CrossRef]
- Lorente, D.; Castro, E.; Lozano, R.; Puente, J.; Romero-Laorden, N.; Morales-Barrera, R.; Rodriguez Vida, A.; Sáez, M.I.; Mendez-Vidal, M.J.; Fernandez, E.; et al. Correlation between time to PSA progression (TTPP), radiographic progression-free survival (rPFS) and overall survival (OS) in first-line abiraterone/enzalutamide (Abi/Enza) and docetaxel (Doc) treated patients in a prospective cohort study. J. Clin. Oncol. 2019, 37, 267. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Hussain, M.; Wolf, M.; Marshall, E.; Crawford, E.D.; Eisenberger, M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: A Southwest Oncology Group report. J. Clin. Oncol. 1994, 12, 1868–1875. [Google Scholar] [CrossRef]
- Taylor, C.D.; Elson, P.; Trump, D.L. Importance of continued testicular suppression in hormone-refractory prostate cancer. J. Clin. Oncol. 1993, 11, 2167–2172. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ryan, C.J.; Shah, S.; Efstathiou, E.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Logothetis, C.J.; Kheoh, T.; Kilian, C.; Haqq, C.M.; et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res. 2011, 17, 4854–4861. [Google Scholar] [CrossRef]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2017, 25, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.N.; Annala, M.; Sunderland, K.; Khalaf, D.; Finch, D.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Beja, K.; Vandekerkhove, G.; et al. A randomized phase II cross-over study of abiraterone + prednisone (ABI) vs. enzalutamide (ENZ) for patients (pts) with metastatic, castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 5002. [Google Scholar] [CrossRef]
- Qu, F.; Xie, W.; Nakabayashi, M.; Zhang, H.; Jeong, S.H.; Wang, X.; Komura, K.; Sweeney, C.J.; Sartor, O.; Lee, G.M.; et al. Association of AR-V7 and Prostate-Specific Antigen RNA Levels in Blood with Efficacy of Abiraterone Acetate and Enzalutamide Treatment in Men with Prostate Cancer. Clin. Cancer Res. 2017, 23, 726–734. [Google Scholar] [CrossRef]




| Variables | Total (n = 184) | Abi (n = 92) | Enz (n = 92) | p Value |
|---|---|---|---|---|
| Age (mean ± SD) | 73.5 + 7.8 | 74.0 + 8.0 | 73.0 + 7.6 | 0.355 |
| SRE during follow-up | ||||
| No (%) | 144 (78.3) | 72 (78.3) | 72 (78.3) | |
| Yes (%) | 40 (21.7) | 20 (21.7) | 20 (21.7) | 1.000 |
| Taxanes during follow-up | ||||
| No (%) | 159 (86.4) | 80 (87.0) | 79 (85.9) | |
| Yes (%) | 25 (13.6) | 12 (13.0) | 13 (14.1) | 0.809 |
| ADT response duration | ||||
| ≥12months (%) | 136 (73.9) | 65 (70.6) | 71 (77.2) | |
| <12 months (%) | 48 (26.1) | 27 (29.4) | 21 (22.8) | 0.809 |
| Median PSA level at diagnosis (ng/mL) (quartile) | 124.0 (29.3, 395.6) | 124.3 (41.9, 327.1) | 93.8 (25.7, 574.8) | 0.685 |
| Median PSA level at first line treatment (ng/mL) (quartile) | 6.8 (2.3, 30.1) | 6.8 (2.0, 30.4) | 6.8 (2.5, 30.1) | 0.918 |
| Gleason sum score (%) | ||||
| ≤7 | 20 (10.9) | 9 (9.8) | 11 (12.0) | |
| 8 | 41 (22.3) | 20 (21.7) | 21 (22.8) | |
| 9 | 113 (61.4) | 56 (60.9) | 57 (62.0) | |
| 10 | 10 (5.4) | 7 (7.6) | 3 (3.3) | 0.598 |
| Local treatment prior to ADT (%) | ||||
| Non | 156 (84.8) | 77 (83.7) | 79 (85.9) | |
| Prostatectomy | 16 (8.7) | 9 (9.8) | 7 (7.6) | |
| Radiation | 8 (4.3) | 5 (5.4) | 3 (3.3) | |
| Others | 4 (2.2) | 1 (1.1) | 3 (3.3) | 0.311 |
| Initial ADT (%) | ||||
| LHRH analog + NAs | 162 (88.0) | 78 (84.8) | 84 (91.3) | |
| LHRH analog | 11 (6.0) | 7 (7.6) | 4 (4.3) | |
| NAs | 7 (3.8) | 5 (5.4) | 2 (2.2) | |
| Others | 4 (2.2) | 2 (2.2) | 2 (2.2) | 0.113 |
| Mets at first line treatment (%) | ||||
| M0 | 57 (31.0) | 27 (29.4) | 30 (32.6) | |
| M1 | 127 (69.0) | 65 (70.7) | 62 (67.4) | 0.345 |
| Visceral mets at first line treatment (%) | ||||
| No | 165 (89.7) | 82 (89.1) | 83 (90.2) | |
| Yes | 19 (10.3) | 10 (10.9) | 9 (9.8) | 0.809 |
| LN mets at first line treatment (%) | ||||
| Non | 117 (63.6) | 55 (59.8) | 62 (67.4) | |
| Regional | 45 (24.5) | 23 (25.0) | 22 (23.9) | |
| Non-regional | 22 (12.0) | 14 (15.2) | 8 (8.7) | 0.350 |
| No. of bone mets at first line treatment (%) | ||||
| 0 | 83 (45.1) | 40 (43.5) | 43 (46.7) | |
| 1 | 23 (12.5) | 11 (12.0) | 12 (13.0) | |
| 2 | 15 (8.2) | 8 (8.7) | 7 (7.6) | |
| >3 | 63 (34.2) | 33 (35.9) | 30 (32.6) | 0.948 |
| ECOG-PS (%) | ||||
| 0 | 104 (56.5) | 51 (55.4) | 53 (57.6) | |
| 1 | 66 (35.9) | 35 (38.0) | 31 (33.7) | |
| ≥2 | 14 (7.6) | 6 (6.6) | 8 (8.7) | 0.684 |
| Neutrophil-lymphocyte ratio at first line treatment (mean ± SD) | 2.99 ± 2.49 | 3.08 ± 2.93 | 2.84 ± 1.59 | 0.648 |
| Hb at first line treatment (g/dL) (mean ± SD) | 12.2 ± 1.8 | 12.1 ± 1.8 | 12.3 ± 1.8 | 0.627 |
| Platelet count at first line treatment (103/uL) (mean ± SD) | 213 ± 75 | 215 ± 80 | 210 ± 71 | 0.640 |
| ALP at first line treatment (U/L) (quartile) | 248 (202, 350) | 252 (199, 370) | 246 (213, 344) | 0.884 |
| LDH at first line treatment (U/L) (quartile) | 200 (182, 238) | 201 (177, 231) | 200 (185, 240) | 0.675 |
| Albumin (g/dL) (quartile) | 4.1 (3.8, 4.4) | 4.1 (3.6, 4.3) | 4.2 (3.9, 4.4) | 0.126 |
| CRP (mg/dL) (quartile) | 0.1 (0.05, 0.32) | 0.1 (0.05, 0.23) | 0.1 (0.05, 0.48) | 0.670 |
| Variables | Total (n = 84) | Abi to Enz (n = 46) | Enz to Abi (n = 38) | p Value |
|---|---|---|---|---|
| Age (mean ± SD) | 73.0 + 7.9 | 71.8 + 7.3 | 74.4 + 8.4 | ns |
| SRE during follow-up | ||||
| No (%) | 63 (75.0) | 32 (69.6) | 31 (81.6) | |
| Yes (%) | 21 (25.0) | 14 (30.4) | 7 (18.4) | ns |
| Median PSA level at 2nd line treatment (ng/mL) (quartile) | 21.3 (3.8, 93.2) | 21.5 (8.1, 61.3) | 21.0 (3.4, 94.3) | ns |
| Gleason sum score (%) | ||||
| ≤7 | 11 (13.1) | 8 (17.4) | 3 (7.9) | |
| 8 | 16 (19.0) | 6 (13.0) | 10 (26.3) | |
| 9 | 53 (63.1) | 30 (65.2) | 23 (60.5) | |
| 10 | 4 (4.8) | 2 (4.4) | 2 (5.3) | ns |
| Mets at 2nd line treatment (%) | ||||
| M0 | 16 (19.0) | 9 (19.6) | 7 (18.4) | |
| M1 | 68 (81.0) | 37 (80.4) | 31 (81.6) | ns |
| Visceral mets at 2nd line treatment (%) | ||||
| No | 75 (88.2) | 39 (84.8) | 36 (94.7) | |
| Yes | 9 (11.8) | 7 (15.2) | 2 (5.3) | ns |
| LN mets at 2nd line treatment (%) | ||||
| No | 59 (70.2) | 31 (67.4) | 28 (73.7) | |
| Yes | 25 (29.8) | 15 (32.6) | 10 (26.3) | ns |
| Bone mets at 2nd line treatment (%) | ||||
| No | 25 (29.8) | 14 (30.4) | 11 (29.0) | |
| Yes | 59 (70.2) | 32 (69.6) | 27 (71.1) | ns |
| Taxanes during follow-up | ||||
| No | 72 (85.7) | 41 (89.1) | 30 (79.0) | |
| Yes | 12 (14.3) | 5 (10.9) | 8 (21.0) | ns |
| ECOG-PS (%) | ||||
| 0 | 34 (40.5) | 17 (37.0) | 17 (44.7) | |
| 1 | 45 (53.6) | 27 (58.7) | 18 (47.4) | |
| ≥2 | 5 (5.9) | 2 (4.4) | 3 (7.9) | ns |
| PSA decline ≥50% at first line treatment | ||||
| No (%) | 43 (51.2) | 28 (60.9) | 15 (39.5) | |
| Yes (%) | 41 (48.8) | 18 (39.1) | 23 (60.5) | 0.037 |
| TTPP at 2nd Line | Radiographic PFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p Value | HR | 95%CI | p Value | ||
| Treatment sequence | ||||||||
| Abi to Enz | Ref | Ref | ||||||
| Enz to Abi | 1.791 | 1.091 | 3.163 | 0.043 | 1.538 | 0.855 | 3.019 | 0.219 |
| Visceral mets at 2nd line treatment | ||||||||
| No | ||||||||
| Yes | 3.647 | 1.003 | 23.634 | 0.049 | 3.647 | 1.182 | 19.278 | 0.032 |
| LN mets at 2nd line treatment | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.663 | 0.847 | 3.406 | 0.141 | 1.233 | 0.784 | 2.392 | 0.221 |
| Bone mets at 2nd line treatment | ||||||||
| No | Ref | Ref | ||||||
| Yes | 1.946 | 0.972 | 4.071 | 0.06 | 1.392 | 0.872 | 4.281 | 0.099 |
| PSA decline ≥50% at first line | ||||||||
| No | Ref | Ref | ||||||
| Yes | 0.641 | 0.401 | 0.933 | 0.038 | 0.865 | 0.431 | 1.283 | 0.492 |
| ECOG-PS | ||||||||
| 0 | Ref | Ref | ||||||
| >1 | 2.154 | 1.163 | 4.154 | 0.014 | 1.538 | 0.699 | 2.193 | 0.293 |
| Variables | Spearman’s Correlation Coefficient (SCC) (95%CI) | p Value |
|---|---|---|
| rPFS | 0.601 (0.411–0.722) | <0.001 |
| TTPP | 0.468 (0.275–0.625) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komura, K.; Fujiwara, Y.; Uchimoto, T.; Saito, K.; Tanda, N.; Matsunaga, T.; Ichihashi, A.; Tsutsumi, T.; Tsujino, T.; Yoshikawa, Y.; et al. Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. J. Clin. Med. 2019, 8, 1251. https://doi.org/10.3390/jcm8081251
Komura K, Fujiwara Y, Uchimoto T, Saito K, Tanda N, Matsunaga T, Ichihashi A, Tsutsumi T, Tsujino T, Yoshikawa Y, et al. Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. Journal of Clinical Medicine. 2019; 8(8):1251. https://doi.org/10.3390/jcm8081251
Chicago/Turabian StyleKomura, Kazumasa, Yuya Fujiwara, Taizo Uchimoto, Kenkichi Saito, Naoki Tanda, Tomohisa Matsunaga, Atsushi Ichihashi, Takeshi Tsutsumi, Takuya Tsujino, Yuki Yoshikawa, and et al. 2019. "Comparison of Radiographic Progression-Free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort" Journal of Clinical Medicine 8, no. 8: 1251. https://doi.org/10.3390/jcm8081251