Can NF-κB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View
Abstract
:1. Introduction
2. NF-κB and Oncogenesis
3. NF-κB in Multi-Drug Resistant Human Tumor
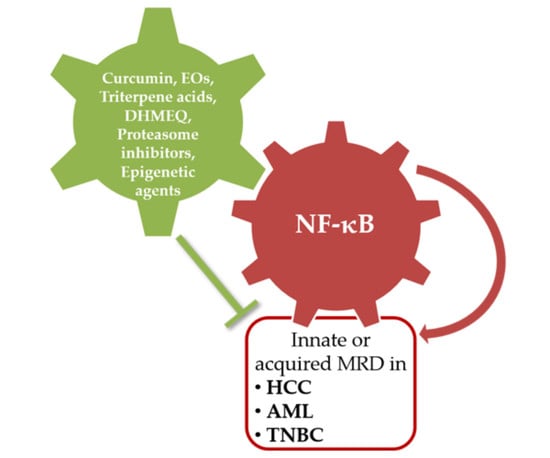
4. NF-κB as Molecular Drug Target
4.1. Natural Compounds
4.1.1. Curcumin
4.1.2. Essential Oils
4.1.3. Triterpene Acids
4.2. Synthetic Compounds
4.2.1. Dehydroxymethylepoxyquinomicin (DHMEQ): A Selective Inhibitor of NF-κB
4.2.2. Proteasome Inhibitor
4.2.3. Epigenetic Agents
4.2.4. Silencing of MDA-9/Syntenin
5. Other Scientific Evidence
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, T.D.; Kalaitzidis, D.; Liang, M.C.; Starczynowsk, D.T. The c-Rel transcription factor and B-cell proliferation: A deal with the devil. Oncogene 2004, 23, 2275–2286. [Google Scholar] [CrossRef] [Green Version]
- Braun, T.; Carvalho, G.; Coquelle, A.; Vozenin, M.C.; Lepelley, P.; Hirsch, F.; Kiladjian, J.J.; Ribrag, V.; Fenaux, P.; Kroemer, G. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood 2006, 107, 1156–1165. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, C.M.; Stavnes, H.T.; Kleinberg, L.; Berner, A.; Hernandez, L.F.; Birrer, M.J.; Steinberg, S.M.; Davidson, B.; Kohn, E.C. Nuclear factor κB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer 2010, 116, 3276–3284. [Google Scholar] [CrossRef] [Green Version]
- Codony-Servat, J.; Marín-Aguilera, M.; Visa, L.; García-Albéniz, X.; Pineda, E.; Fernández, P.L.; Filella, X.; Gascón, P.; Mellado, B. Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castration-resistant prostate cancer. Prostate 2013, 73, 512–552. [Google Scholar] [CrossRef]
- Long, Y.M.; Ye, S.; Rong, J.; Xie, W.R. Nuclear factor kappa B: A marker of chemotherapy for human stage IV gastric carcinoma. World J. Gastroenterol. 2008, 14, 4739–4744. [Google Scholar] [CrossRef]
- Plewka, D.; Plewka, A.; Miskiewicz, A.; Morek, M.; Bogunia, E. Nuclear factor-kappa B as potential therapeutic target in human colon cancer. J. Cancer Res. Ther. 2018, 14, 516–520. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets, Inflammation and Liver Cancer. Semin. Liver Dis. 2019, 39, 26–42. [Google Scholar] [CrossRef]
- Prajoko, Y.W.; Aryandono, T. Expression of nuclear factor kappa B (NF-kB) as a predictor of poor pathologic response to chemotherapy in patients with locally advanced breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zu, X.; Liu, K.; Bode, A.M.; Dong, Z.; Liu, Z.; Kim, D.J. Knockdown of Pyruvate Kinase M Inhibits Cell Growth and Migration by Reducing NF-kB Activity in Triple-Negative Breast Cancer Cells. Mol Cells 2019, 42, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Basseres, D.S.; Baldwin, A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 9, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.P.; Bours, V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Burstein, E.; Duckett, C.S. Dying for NF-kappaB? Control of cell death by transcriptional regulation of the apoptotic machinery. Curr. Opin. Cell Biol. 2003, 15, 732–737. [Google Scholar] [CrossRef]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gélinas, C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Pettaway, C.A.; Uehara, H.; Bucana, C.D.; Fidler, I.J. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 2001, 20, 4188–4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.A.; Beug, H.; Wirth, T. Epithelial-mesenchymal transition: NF-kappaB takes center stage. Cell Cycle 2004, 3, 1477–1480. [Google Scholar] [CrossRef] [Green Version]
- Boukerche, H.; Su, Z.Z.; Emdad, L.; Sarkar, D.; Fisher, P.B. mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB. Cancer Res. 2007, 67, 1812–1822. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, S.; Das, S.K.; Pradhan, A.K.; Emdad, L.; Windle, J.J.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin (SDCBP) Is a Critical Regulator of Chemoresistance, Survival and Stemness in Prostate Cancer Stem Cells. Cancers (Basel) 2019, 23, 53. [Google Scholar] [CrossRef] [Green Version]
- Bhoopathi, P.; Pradhan, A.K.; Bacolod, M.D.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene 2019, 38, 6781–6793. [Google Scholar] [CrossRef]
- Das, S.K.; Sarkar, D.; Cavenee, W.K.; Emdad, L.; Fisher, P.B. Rethinking Glioblastoma Therapy: MDA-9/Syntenin Targeted Small Molecule. ACS Chem. Neurosci. 2019, 10, 1121–1123. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Maji, S.; Wechman, S.L.; Bhoopathi, P.; Pradhan, A.K.; Talukdar, S.; Sarkar, D.; Landry, J.; Guo, C.; Wang, X.Y.; et al. MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol. Res. 2020, 13, 104695. [Google Scholar] [CrossRef]
- Bednarski, B.K.; Ding, X.; Coombe, K.; Baldwin, A.S.; Kim, H.J. Active roles for inhibitory kappaB kinases alpha and beta in nuclear factor-kappaB-mediated chemoresistance to doxorubicin. Mol. Cancer Ther. 2008, 7, 1827–1835. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, L.; Ni, Z.; Sun, J.; Gao, H.; Cheng, Z.; Xu, J.; Yin, P. Resveratrol induces AMPK-dependent MDR1 inhibition in colorectal cancer HCT116/L-OHP cells by preventing activation of NF-κB signaling and suppressing cAMP-responsive element transcriptional activity. Tumor Biol. 2015, 36, 9499–9510. [Google Scholar] [CrossRef]
- Suttana, W.; Mankhetkorn, S.; Poompimon, W.; Palagani, A.; Zhokhov, S.; Gerlo, S.; Haegeman, G.; Berghe, W.V. Differential chemosensitization of P-glycoprotein overexpressing K562/Adr cells by withaferin A and Siamois polyphenols. Mol. Cancer 2010, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.Z.; Ni, K.; Guo, C.; Zhang, C.; Geng, Y.D.; Wang, Z.D.; Yang, L.; Kong, L.Y. Alopecurone B reverses doxorubicin-resistant human osteosarcoma cell line by inhibiting P-glycoprotein and NF-kappa B signaling. Phytomedicine 2015, 22, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Filipits, M. Mechanisms of cancer: Multidrug resistance. Drug Discov. Today Dis. Mech. 2004, 2, 229–234. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Advani, P.; Moreno-Aspitia, A. Current strategies for the prevention of breast cancer. Breast Cancer 2014, 6, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmadeka, R.; Harmon, B.E.; Singh, M. Triple-negative breast carcinoma: Current and emerging concepts. Am. J. Clin. Pathol. 2014, 141, 462–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russnes, H.G.; Navin, N.; Hicks, J.; Borresen-Dale, A.L. Insight into the heterogeneity of breast cancer through next-generation sequencing. J. Clin. Investig. 2011, 121, 3810–3818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.; Sarkar, F.H.; Ahmed, Q.; Bandyopadhyay, S.; Nahleh, Z.A.; Semaan, A.; Sakr, W.; Munkarah, A.; Ali-Fehmi, R. Molecular markers of epithelial-to-mesenchymal transition are associated with tumor aggressiveness in breast carcinoma. Transl. Oncol. 2011, 4, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Piao, H.L.; Yuan, Y.; Wang, M.; Sun, Y.; Liang, H.; Ma, L. α-catenin acts as a tumour suppressor in E-cadherin-negative basal-like breast cancer by inhibiting NF-κB signalling. Nat. Cell Biol. 2014, 16, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Barbie, T.U.; Alexe, G.; Aref, A.R.; Li, S.; Zhu, Z.; Zhang, X.; Imamura, Y.; Thai, T.C.; Huang, Y.; Bowden, M.; et al. Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J. Clin. Investig. 2014, 124, 5411–5423. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ito, T.; Azuma, S.; Ito, E.; Honma, R.; Yanagisawa, Y.; Nishikawa, A.; Kawamura, M.; Imai, J.; Watanabe, S.; et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci. 2009, 100, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.; Wang, Y.; Budoff, A.; Xu, Q.; Lituev, A.; Potapova, O.; Vansant, G.; Monforte, J.; Daraselia, N. Exploring molecular pathways of triple-negative breast cancer. Genes Cancer 2011, 2, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.C.; Guttridge, D.C.; Funkhouser, W.K.; Baldwin, A.S., Jr. Selective activation of NF-kappa B subunits in human breast cancer: Potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 2000, 19, 1123–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.T.; Li, Z.; Wu, Z.; Aau, M.; Guan, P.; Karuturi, R.K.; Liou, Y.C.; Yu, Q. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol. Cell 2011, 43, 798–810. [Google Scholar] [CrossRef]
- D’Alessandro, N.; Poma, P.; Labbozzetta, M.; Vivona, N.; Notarbartolo, M. Mechanisms of Raf-1 Kinase Inhibitor Protein Dysregulation in Triple-Negative Breast Cancers and Identification of Possible Novel Therapeutic Approaches for These Tumors. Forum Immunopathol. Dis. Ther. 2014, 5, 215–222. [Google Scholar] [CrossRef]
- Poma, P.; Notarbartolo, M.; Labbozzetta, M.; Maurici, A.; Carina, V.; Alaimo, A.; Rizzi, M.; Simoni, D.; D’Alessandro, N. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: Analysis of the possible molecular basis. Int. J. Mol. Med. 2007, 20, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Poma, P.; Labbozzetta, M.; D’Alessandro, N.; Notarbartolo, M. NF-κB Is a Potential Molecular Drug Target in Triple-Negative Breast Cancers. OMICS 2017, 21, 225–231. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple Negative Breast Cancer Cell Lines: One Tool in the Search for Better Treatment of Triple Negative Breast Cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, B.S.; Sharma, S.C.; Chanana, P.; Jhamb, S. Systemic treatment strategies for triple-negative breast cancer. World J. Clin. Oncol. 2014, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Abramson, V.G.; Lehmann, B.D.; Ballinger, T.J.; Pietenpol, J.A. Subtyping of triple-negative breast cancer: Implications for therapy. Cancer 2015, 121, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graffenried, L.A.; Chandrasekar, B.; Friedrichs, W.E.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.W.; Weiss, G.R. NF-kB inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann. Oncol. 2004, 15, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Haybaeck, J.; Zeller, N.; Wolf, M.J.; Weber, A.; Wagner, U.; Kurrer, M.O.; Bremer, J.; Iezzi, G.; Graf, R.; Clavien, P.A.; et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009, 16, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Kimmoun, E.; Legrand, A.; Sauvanet, A.; Degott, C.; Lardeux, B.; Bernuau, D. Activation of NF-kappaB, AP-1 and STAT transcription factors is a frequent and early event in human hepatocellular carcinomas. J. Hepatol. 2002, 37, 63–71. [Google Scholar] [CrossRef]
- Arsura, M.; Cavin, L.G. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005, 229, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Vainer, G.W.; Pikarsky, E.; Ben-Neriah, Y. Contradictory functions of NF-kB in liver physiology and cancer. Cancer Lett. 2008, 267, 182–188. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Beraza, N.; Kotsikoris, V.; van Loo, G.; Nenci, A.; De Vos, R.; Roskams, T.; Trautwein, C.; Pasparakis, M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007, 11, 119–132. [Google Scholar] [CrossRef]
- Sunami, Y.; Ringelhan, M.; Kokai, E.; Lu, M.; O’Connor, T.; Lorentzen, A.; Weber, A.; Rodewald, A.K.; Mullhaupt, B.; Terracciano, L.; et al. Canonical NF-kappaB signaling in hepatocytes acts as a tumor-suppressor in hepatitis B virus surface antigen-driven hepatocellular carcinoma by controlling the unfolded protein response. Hepatology 2016, 63, 1592–1607. [Google Scholar] [CrossRef]
- Chiao, P.J.; Na, R.; Niu, J.; Sclabas, G.M.; Dong, Q.; Curley, S.A. Role of Rel/NF-kappaB transcription factors in apoptosis of human hepatocellular carcinoma cells. Cancer 2002, 95, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Canc. 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 224, 53–65. [Google Scholar] [CrossRef]
- Al-Bahar, S.; Adriana, Z.; Pandita, R. Acute myeloid leukemia as a genetic disease. Gulf J. Oncol. 2008, 3, 9–15. [Google Scholar] [PubMed]
- Zhou, J.; Ching, Y.Q.; Chng, W.J. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: From molecular pathogenesis to therapeutic target. Oncotarget 2015, 6, 5490–5500. [Google Scholar] [CrossRef] [Green Version]
- Grandage, V.L.; Gale, R.E.; Linch, D.C.; Khwaja, A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NFkB, MAPkinase and p53 pathways. Leukemia 2005, 19, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Li, Y.; Zhang, H.Y.; Zheng, Y.; Liu, X.L.; Hu, Z.; Wang, Y.; Wang, J.; Cai, Y.H.; Liu, Q.; et al. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget 2017, 8, 89643–89654. [Google Scholar] [CrossRef] [Green Version]
- Cilloni, D.; Martinelli, G.; Messa, F.; Baccarani, M.; Saglio, G. Nuclear factor k B as a target for new drug development in myeloid malignancies. Haematologica 2007, 92, 1224–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef]
- Brandao, M.M.; Soares, E.; Salles, T.S.; Saad, S.T. Expression of inducible nitric oxide synthase is increased in acute myeloid leukaemia. Acta Haematol. 2001, 106, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hjortso, M.D.; Andersen, M.H. The expression, function and targeting of haem oxygenase-1 in cancer. Curr. Cancer Drug Targets 2014, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 2008, 111, 3793–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, B.; Weber, M.; Quirling, M.; Fischer, C.; Page, S.; Adam, M.; Von Schilling, C.; Waterhouse, C.; Schmid, C.; Neumeier, D.; et al. Increased IkappaB kinase activity is associated with activated NF-kappaB in acute myeloid blasts. Leukemia 2002, 16, 2062–2071. [Google Scholar] [CrossRef] [Green Version]
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Poma, P.; Dusonchet, L.; Meli, M.; D’Alessandro, N. Expression of the IAPs in multidrug resistant tumor cells. Oncol. Rep. 2004, 11, 133–136. [Google Scholar] [CrossRef]
- Tamm, I.; Kornblau, S.M.; Segall, H.; Krajewski, S.; Welsh, K.; Kitada, S.; Scudiero, D.A.; Tudor, G.; Qui, Y.H.; Monks, A.; et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin. Cancer Res. 2000, 6, 1796–1803. [Google Scholar]
- Hrdinka, M.; Yabal, M. Inhibitor of apoptosis proteins in human health and disease. Genes Immun. 2019, 20, 641–650. [Google Scholar] [CrossRef]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Bharali, D.J.; Elbaz, O.; El-Ghaffar, H.A.A.; Azmy, E.; Anber, N.; Mousa, S.A. Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia. Molecule. 2019, 24, 2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, A.; Zukerman, T.; Flaishon, L.; Yakar, R.B.; Rowe, J.M. Efficacy outcomes in the treatment of older or medically unfit patients with acute myeloid leukaemia: A systematic review and meta-analysis. Leuk. Res. 2019, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M. Inhibition of survival signalling by dietary polyphenols and indole-3-carbinol. Eur. J. Cancer 2005, 41, 1842–1853. [Google Scholar] [CrossRef]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef]
- Shishodia, S.; Potdar, P.; Gairola, C.G.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 2003, 24, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 3, 363–398. [Google Scholar]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P.; Maurici, A.; Inguglia, L.; Marchetti, P.; Rizzi, M.; Baruchello, R.; Simoni, D.; D’Alessandro, N. Curcumin as a possible lead compound against hormone-indipendent multidrug resistant breast cancer. Ann. N. Y. Acad. Sci. 2009, 1115, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P.; Durand-Fontanier, S.; Delage, C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Robinson, T.P.; Ehlers, T.; Hubbard, R.B.; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorg. Med. Chem. Lett. 2003, 13, 115–117. [Google Scholar] [CrossRef]
- Mosley, C.A.; Liotta, D.C.; Snyder, J.P. Highly active anticancer curcumin analogues. Adv. Exp. Med. Biol. 2007, 595, 77–103. [Google Scholar] [CrossRef]
- Simoni, D.; Rizzi, M.; Rondanin, R.; Baruchello, R.; Marchetti, P.; Invidiata, F.P.; Labbozzetta, M.; Poma, P.; Carina, V.; Notarbartolo, M.; et al. Antitumor effects of curcumin and structurally b-diketone modified analogs on multidrug resistant cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 845–849. [Google Scholar] [CrossRef]
- De Vreese, R.; Grootaert, C.; D’hoore, S.; Theppawong, A.; Van Damme, S.; Van Bogaert, M.; Van Camp, J.; D’hooghe, M. Synthesis of novel curcuminoids accommodating a central β-enaminone motif and their impact on cell growth and oxidative stress. Eur. J. Med. Chem. 2016, 123, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Abcouwer, S.F.; Deck, L.M.; Vander Jagt, D.L. Anti-oxidant activities of curcumin and related enones. Bioorg. Med. Chem. 2005, 13, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.M.; Labbozzetta, M.; Barattucci, A.; Salerno, T.M.G.; Poma, P.; Notarbartolo, M. Synthesis of Curcumin Derivatives and Analysis of Their Antitumor Effects in Triple Negative Breast Cancer (TNBC) Cell Lines. Pharmaceuticals 2019, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limtrakul, P. Curcumin as a chemosensitizer. Adv. Exp. Med. Biol. 2007, 595, 269–300. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of itsdegradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Amano, C.; Minematsu, H.; Fujita, K.; Iwashita, S.; Adachi, M.; Igarashi, K.; Hinuma, S. Nanoparticles containing curcumin useful for suppressing macrophages in vivo in mice. PLoS ONE 2015, 10, e0137207. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.K.H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf. B Biointerfaces 2016, 140, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Poma, P.; Colletti, C.G.; Barattucci, A.; Bonaccorsi, P.M.; Lazzara, G.; Nicotra, G.; Parisi, F.; Salerno, T.M.G.; Spinella, C.; et al. Chemical and biological evaluation of cross-linked halloysite-curcumin derivatives. Appl. Clay Sci. 2020, 184, 105400. [Google Scholar] [CrossRef]
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Crellin, J.K.; Philpott, J.; Bass, A.L.T. Herbal Medicine Past and Present: A Reference Guide to Medicinal Plants; Duke University Press: Durham, NC, USA, 1990. [Google Scholar]
- Fernandes, F.; Andrade, P.B.; Ferreres, F.; Gil-Izquierdo, A.; Sousa-Pinto, I.; Valentão, P. The chemical composition on fingerprint of Glandora diffusa and its biological properties. Arabian J Chem. 2017, 10, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Milad, R.; El-Ahmady, S.; Singab, A.N. Genus Kalanchoe (Crassulaceae): A review of its ethnomedicinal, botanical, chemical and pharmacological properties. Eur. J. Med. Plants 2014, 4, 86–104. [Google Scholar] [CrossRef]
- Zito, P.; Labbozzetta, M.; Notarbartolo, M.; Sajeva, M.; Poma, P. Essential oil of Cyphostemma juttae (Vitaceae): Chemical composition and antitumor mechanism in triple negative breast cancer cells. PLoS ONE 2019, 14, e0214594. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; McCubrey, J.A.; Ramarosandratana, A.V.; Sajeva, M.; Zito, P.; Notarbartolo, M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals 2019, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential Oil Composition of Alluaudia procera and in Vitro Biological Activity on Two Drug-Resistant Models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef] [Green Version]
- Poma, P.; Labbozzetta, M.; Notarbartolo, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Sajeva, M.; Zito, P. Chemical composition, in vitro antitumor and pro-oxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS ONE 2018, 13, e0196947. [Google Scholar] [CrossRef]
- Paszel-Jaworska, A.; Romaniuk, A.; Rybczynska, M. Molecular mechanisms of biological activity of oleanolic acid—A source of inspiration for a new drugs design. Mini-Rev. Org. Chem. 2014, 11, 330–342. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.Q.; Liu, D.; Cai, L.L.; Chen, H.; Cao, B.; Wang, Y.Z. The synthesis of ursolic acid derivatives with cytotoxic activity and the investigation of their preliminary mechanism of action. Biorg. Med. Chem. 2009, 17, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Svarcova, I.; Heinrich, J.; Valentova, K. Berry fruits as a source of biologically active compounds: The case of Lonicera caerulea. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2007, 151, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Quesada, C.; Lopez-Biedma, A.; Warleta, F.; Campos, M.; Beltran, G.; Gaforio, J.J. Bioactive properties of the main Triterpenes found in Olives, Virgin Olive Oil, and Leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Katsman, A.; Umezawa, K.; Bonavida, B. Chemosensitization and immunosensitization of resistant cancer cells to apoptosis and inhibition of metastasis by the specific NF-kappaB inhibitor DHMEQ. Curr. Pharm. De.s 2009, 15, 792–808. [Google Scholar] [CrossRef]
- Ukaji, T.; Umezawa, K. Novel approaches to target NF-κB and other signaling pathways in cancer stem cells. Adv. Biol. Regul. 2014, 56, 108–115. [Google Scholar] [CrossRef]
- Lin, Y.; Ukaji, T.; Koide, N.; Umezawa, K. Inhibition of Late and Early Phases of Cancer Metastasis by the NF-κB Inhibitor DHMEQ Derived from Microbial Bioactive Metabolite Epoxyquinomicin: A Review. Int. J. Mol. Sci. 2018, 19, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; He, H.; Xie, Z.; Wen, H.; Li, X.; Li, X.; Ma, J.; Umezawa, K.; Zhang, Y. Dehydroxymethylepoxyquinomicin suppresses atopic dermatitis-like lesions in a stratum corneum-removed murine model through NF-κB inhibition. Immunopharmacol. Immunotoxicol. 2019, 41, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Keino, H.; Kudo, A.; Hirakata, A.; Okada, A.A.; Umezawa, K. Anti-Inflammatory Effect of Dehydroxymethylepoxyquinomicin, a Nuclear factor-κB Inhibitor, on Endotoxin-Induced Uveitis in Rats In vivo and In vitro. Ocul. Immunol. Inflamm. 2020, 28, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Notarbartolo, M.; Labbozzetta, M.; Sanguedolce, R.; Alaimo, A.; Carina, V.; Maurici, A.; Cusimano, A.; Cervello, M.; D’Alessandro, N. Antitumor effects of the novel NF-kappaB inhibitor dehydroxymethyl-epoxyquinomicin on human hepatic cancer cells: Analysis of synergy with cisplatin and of possible correlation with inhibition of pro-survival genes and IL-6 production. Int. J. Oncol. 2006, 28, 923–930. [Google Scholar]
- Qureshi, A.A.; Zuvanich, E.G.; Khan, D.A.; Mushtaq, S.; Silswal, N.; Qureshi, N. Proteasome inhibitors modulate anticancer and anti-proliferative properties via NF-kB signaling, and ubiquitin-proteasome pathways in cancer cell lines of different organs. Lipids Health Dis. 2018, 17, 62. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Cai, C.Y.; Assaraf, Y.G.; Guo, H.Q.; Cui, Q.; Wei, L.; Huang, J.J.; Ashby, C.R., Jr.; Chen, Z.S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updat. 2019, 48, 100663. [Google Scholar] [CrossRef]
- Shalem-Cohavi, N.; Beery, E.; Nordenberg, J.; Rozovski, U.; Raanani, P.; Lahav, M.; Uziel, O. The Effects of Proteasome Inhibitors on Telomerase Activity and Regulation in Multiple Myeloma Cells. Int J Mol Sci 2019, 20, 2509. [Google Scholar] [CrossRef] [Green Version]
- Minn, A.J.; Bevilacqua, E.; Yun, J.; Rosner, M.R. Identification of novel metastasis suppressor signaling pathways for breast cancer. Cell Cycle 2012, 11, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Yeung, K.; Seitz, T.; Li, S.; Janosch, P.; McFerran, B.; Kaiser, C.; Fee, F.; Katsanakis, K.D.; Rose, D.W.; Mischak, H.; et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 1999, 401, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Schuierer, M.M.; Bataille, F.; Weiss, T.S.; Hellerbrand, C.; Bosserhoff, A.K. Raf kinase inhibitor protein is downregulated in hepatocellular carcinoma. Oncol. Rep. 2006, 16, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Bonavida, B.; Chatzaki, E.; Baritaki, S. RKIP: A Key Regulator in Tumor Metastasis Initiation and Resistance to Apoptosis: Therapeutic Targeting and Impact. Cancers (Basel) 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odabaei, G.; Chatterjee, D.; Jazirehi, A.R.; Goodglick, L.; Yeung, K.; Bonavida, B. Raf-1 kinase inhibitor protein: Structure, function, regulation of cell signaling and pivotal role in apoptosis. Adv. Cancer Res. 2004, 91, 169–200. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Park, S.; Sun, S.C.; Trumbly, R.; Ren, G.; Tsung, E.; Yeung, K.C. RKIP inhibits NF-kappaB in cancer cells by regulating upstream signaling components of the IkappaB kinase complex. FEBS Lett. 2010, 584, 662–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Bonavida, B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit. Rev. Immunol. 2009, 29, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.H.; Gao, X.; Yu, B.; Qiao, H. Raf Kinase Inhibitor Protein (RKIP) Inhibits Tumor Necrosis Factor-α (TNF-α) Induced Adhesion Molecules Expression in Vascular Smooth Muscle Bells by Suppressing (Nuclear Transcription Factor-κB (NF-kappaB) Pathway. Med. Sci. Monit. 2017, 23, 4789–4797. [Google Scholar] [CrossRef] [Green Version]
- Labbozzetta, M.; Poma, P.; Vivona, N.; Gulino, A.; D’Alessandro, N.; Notarbartolo, M. Epigenetic changes and nuclear factor-κB activation, but not microRNA-224, downregulate Raf-1 kinase inhibitor protein in triple-negative breast cancer SUM 159 cells. Oncol. Lett. 2015, 10, 3807–3815. [Google Scholar] [CrossRef] [Green Version]
- Boukerche, H.; Aissaoui, H.; Prévost, C.; Hirbec, H.; Das, S.K.; Su, Z.Z.; Sarkar, D.; Fisher, P.B. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene 2010, 29, 3054–3066. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Bhutia, S.K.; Sokhi, U.K.; Azab, B.; Su, Z.Z.; Boukerche, H.; Anwar, T.; Moen, E.L.; Chatterjee, D.; Pellecchia, M.; et al. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012, 72, 6217–6226. [Google Scholar] [CrossRef] [Green Version]
- Notarbartolo, M.; Labbozzetta, M.; Pojero, F.; D’Alessandro, N.; Poma, P. Potential Therapeutic Applications of MDA-9/Syntenin-NF-κB-RKIP Loop in Human Liver Carcinoma. Curr. Mol. Med. 2018, 18, 630–639. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Silva, R.C.M.C.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two Sides of the Same Coin. Genes (Basel) 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, A.G. Rationale for combining immunotherapy with chemotherapy. Immunotherapy 2015, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Identifier Number | Official Title | Date | Conclusions |
|---|---|---|---|
| NCT00512798 | (Inhibition of NF-κB Signaling in Melanoma Therapy) A Phase I/II Clinical Trial of PS-341, a Proteasome Inhibitor, in Combination with an Extended Continuous Oral Schedule of Temozolomide in Patients with Advanced Refractory Solid Tumors with the Phase II Component Only in Patients with Melanoma | 2003–2008 | Terminated Due to Lack of Efficacy |
| NCT03047837 | A Randomized, Phase II, Double-Blind, Placebo-controlled, Multicenter, 2x2 Factorial Design Biomarker Tertiary Prevention Trial of Low-dose Aspirin and Metformin in Stage I–III Colorectal Cancer Patients. | 2017–2020 | Not Terminated |
| NCT01681368 | Phase II Open Label Non-Randomized Single Agent Study of the SMAC (Second Mitochondrial-Derived Activator of Caspases)-Mimetic Birinapant (TL32711; NSC 756502) in Relapsed Platinum Resistant or Refractory Epithelial Ovarian Cancer; Primary Peritoneal | 2012–2014 | Terminated Due to Lack of a Clinical Benefit |
| NCT04208334 | A Double-blind, Placebo-Controlled Randomized Trial Phase II Evaluating the Effect of Curcumin for Treatment of Cancer Anorexia–Cachexia Syndrome in Patients with Stage III–IV of Head and Neck Cancer | 2020–2020 | Not Terminated |
| NCT04234022 | Zn-DDC to Target Hypoxia-NF-ĸB–CSCs Pathway and Improve the Treatment Outcomes of Hematological Malignancies—A Translation Bench Study | 2020–2023 | Not Terminated (Not Yet Recruiting) |
| NCT01740323 | Meriva for Treatment-Induced Inflammation and Fatigue in Women with Breast Cancer | 2015–2018 | Completed (the Study Only Highlighted a Slight Advantage by Curcumin Treatment, Compared to Placebo, to Reduce Symptoms of Fatigue Associated with Excessive NF-ĸB Activity in BRCA Patients) |
| NCT00899353 | Inhibition of NF-ĸB for Prevention of Disease Progression in Indolent B Cell Malignancies | 2008–2012 | Terminated (Original Principal Investigator Left the Institution.) |
| NCT02765854 | Phase II Trial of Ixazomib and Dexamethasone Versus Ixazomib, Dexamethasone, and Lenalidomide, Randomized with NF-kB2 Rearrangement (Proteasome Inhibitor NF-kB2 Rearrangement Driven Trial: PINR) | 2016–2021 | Not Terminated |
| NCT01132911 | A Phase I Study of Vorinostat and Bortezomib in Children with Refractory of Recurrent Solid Tumors, Including CNS Tumors and Lymphomas | 2010–2011 | Completed (No Study Results Posted on ClinicalTrials.gov) |
| NCT02144675 | A Randomized Phase II Study of NF-κB Inhibition During Induction Chemotherapy for Patients with Acute Myelogenous Leukemia | 2009–2016 | Completed (It Was Not Reported if the Treatment with Choline Magnesium Trisalicylate and Chemotherapy Produced a Modulation of NF-ĸB Transcriptional Targets and/or Drug Efflux in at Least 50% of Patients) |
| NCT03978624 | Window of Opportunity Platform Study to Define Immunogenomic Changes with Pembrolizumab Alone and in Rational Combinations in Muscle-Invasive Bladder Cancer | 2019–2021 | Not Terminated |
| NCT00280761 | A Biologic Study of Global Gene Expression, NF-Kappa B and p53 in Adenocarcinoma of the Rectum | 2003–2021 | Not Terminated |
| NCT00541359 | A Phase I Trial of PS-341 in Combination with Topotecan in Advanced Solid Tumor Malignancies | 2004–2011 | Completed (No Study Results Posted on ClinicalTrials.gov) |
| NCT00305734 | Phase II Trial of PS-341 (Bortezomib, NSC-681239) Followed by the Addition of Gemcitabine at Progression in Recurrent or Metastatic Nasopharyngeal Carcinoma | 2006–2007 | Completed (No Study Results Posted on ClinicalTrials.gov) |
| NCT00745134 | A Randomized Double Blinded Study of Curcumin with Pre-Operative Capecitabine and Radiation Therapy Followed by Surgery for Rectal Cancer | 2008–2021 | Not Terminated |
| NCT03382340 | A Phase 1/2a Open-Label, Dose-Escalation/Dose-Expansion Safety, Tolerability and Pharmacokinetic Study of IMX-110 (a Nanoparticle Encapsulating a Stat3/NF-kB/Poly-Tyrosine Kinase Inhibitor and Low-Dose Doxorubicin) in Patients with Advanced Solid Tumors | 2018–2021 | Not terminated |
| NCT00113841 | Pilot Study of Curcumin (Diferuloylmethane Derivative) with or without Bioperine in Patients with Multiple Myeloma | 2004–2009 | Completed (Curcumin and bioperine compared to Curcumin Alone Produced a Higher Reduction of NF-ĸB and Its Related Gene Expression in the Multiple Myeloma cells). |
| NCT00305747 | Phase I Study of BioResponse-Dim in Non-Metastatic, Hormone-Refractory Prostate Cancer Patients with Rising Serum PSA | 2005–2010 | Completed (No Study Results Posted on ClinicalTrials.gov) |
| NCT01269203 | A Phase II Randomized Study of the Efficacy of Curcumin for Reducing Symptoms During Maintenance Therapy in Multiple Myeloma Patients | 2012–2015 | Withdrawn |
| NCT00156299 | A Pilot Study of NF-kB Inhibition During Induction Chemotherapy for Patients with Acute Myelogenous Leukemia (AML) | 2003–2008 | Terminated (Replaced by Another Study) |
| NCT02944578 | Biomolecular Effects of Topical Curcumin in HSIL Cervical Neoplasia | 2017–2021 | Not Terminated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-κB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070. https://doi.org/10.3390/ijms21093070
Labbozzetta M, Notarbartolo M, Poma P. Can NF-κB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. International Journal of Molecular Sciences. 2020; 21(9):3070. https://doi.org/10.3390/ijms21093070
Chicago/Turabian StyleLabbozzetta, Manuela, Monica Notarbartolo, and Paola Poma. 2020. "Can NF-κB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View" International Journal of Molecular Sciences 21, no. 9: 3070. https://doi.org/10.3390/ijms21093070