Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro
Abstract
:1. Introduction
2. Results
2.1. Fluoxetine Decreased the Cell Viability and NF-κB Activation of Sk-Hep1 and Hep3B Cells
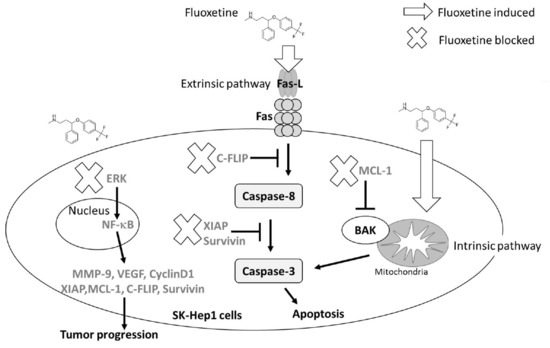
2.2. Fluoxetine Induced Apoptosis and Reduced Expression of Anti-Apoptotic Proteins in SK-Hep1 Cells
2.3. Fluoxetine Promoted Extrinsic and Intrinsic Apoptotic Signaling Transduction in SK-Hep1 and Hep3B Cells
2.4. Fluoxetine Suppressed Cell Migration/Invasion and Reduced ERK Activation and Expression of Metastasis-Associated and Proliferative Proteins in SK-Hep1 and Hep3B Cells
2.5. Fluoxetine Not Only Induced Apoptosis, but Suppressed Tumor Progression in SK-Hep1 and Hep3B Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Antibodies and Reagents
4.2. Cell Culture
4.3. Plasmid Transfection and Stable Clone Selection
4.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
4.5. NF-κB Reporter Gene Assay
4.6. Annexin V/ Propidium Iodide (PI) Staining
4.7. Cell Cycle Analysis
4.8. Measurement of Caspase-3 and -8 Activation
4.9. Measurement of Mitochondria Membrane Potential (ΔΨm)
4.10. Assessment of Fas-L and Fas Activation
4.11. Western Blotting
4.12. Invasion and Migration Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J. Gastroenterol. 2009, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Okada, S.; Ishii, H.; Nose, H.; Nagahama, H.; Nakasuka, H.; Ikeda, K.; Yoshimori, M. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepato-Gastroenterology 1997, 44, 251–257. [Google Scholar] [PubMed]
- Hsu, F.T.; Liu, Y.C.; Chiang, I.T.; Liu, R.S.; Wang, H.E.; Lin, W.J.; Hwang, J.J. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappaB signaling. Int. J. Oncol. 2014, 45, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Ginsburg, A. Cancer-related depression and potential pharmacologic therapies. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon-on-Thames, UK, 2008; Volume 21, pp. 439–441. [Google Scholar]
- Gil-Ad, I.; Zolokov, A.; Lomnitski, L.; Taler, M.; Bar, M.; Luria, D.; Ram, E.; Weizman, A. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int. J. Oncol. 2008, 33, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lieb, J. Defeating cancer with antidepressants. Ecancermedicalscience 2008, 2, 88. [Google Scholar] [CrossRef]
- Fang, C.-K.; Chen, H.-W.; Chiang, I.T.; Chen, C.-C.; Liao, J.-F.; Su, T.-P.; Tung, C.-Y.; Uchitomi, Y.; Hwang, J.-J. Mirtazapine inhibits tumor growth via immune response and serotonergic system. PLoS ONE 2012, 7, e38886. [Google Scholar] [CrossRef]
- Frick, L.R.; Rapanelli, M. Antidepressants: Influence on cancer and immunity? Life Sci. 2013, 92, 525–532. [Google Scholar] [CrossRef]
- Yang, D.K.; Kim, S.J. Desipramine induces apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2017, 38, 1029–1034. [Google Scholar] [CrossRef]
- Kuwahara, J.; Yamada, T.; Egashira, N.; Ueda, M.; Zukeyama, N.; Ushio, S.; Masuda, S. Comparison of the Anti-tumor Effects of Selective Serotonin Reuptake Inhibitors as Well as Serotonin and Norepinephrine Reuptake Inhibitors in Human Hepatocellular Carcinoma Cells. Biol. Pharm. Bull. 2015, 38, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Mun, A.R.; Lee, S.J.; Kim, G.B.; Kang, H.S.; Kim, J.S.; Kim, S.J. Fluoxetine-induced apoptosis in hepatocellular carcinoma cells. Anticancer Res. 2013, 33, 3691–3697. [Google Scholar] [PubMed]
- Rossi, A.; Barraco, A.; Donda, P. Fluoxetine: A review on evidence based medicine. Ann. Gen. Hosp. Psychiatry 2004, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, Y.J.; Jang, E.R.; Kim, W.; Myung, S.C. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin. Pharmacol. Toxicol. 2010, 106, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Stepulak, A.; Rzeski, W.; Sifringer, M.; Brocke, K.; Gratopp, A.; Kupisz, K.; Turski, L.; Ikonomidou, C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol. Ther. 2008, 7, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Duan, J.; Wang, Y.; Chen, X.; Zhou, G.; Wang, R.; Fu, L.; Xu, F. Fluoxetine synergys with anticancer drugs to overcome multidrug resistance in breast cancer cells. Tumour Biol. 2012, 33, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Ito, Y.; Monden, M.; Takeda, T.; Eguchi, H.; Umeshita, K.; Nagano, H.; Nakamori, S.; Dono, K.; Sakon, M.; Nakamura, M.; et al. The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br. J. Cancer 2000, 82, 1211–1217. [Google Scholar] [CrossRef]
- Chiang, I.T.; Chen, W.T.; Tseng, C.W.; Chen, Y.C.; Kuo, Y.C.; Chen, B.J.; Weng, M.C.; Lin, H.J.; Wang, W.S. Hyperforin Inhibits Cell Growth by Inducing Intrinsic and Extrinsic Apoptotic Pathways in Hepatocellular Carcinoma Cells. Anticancer Res. 2017, 37, 161–167. [Google Scholar] [CrossRef]
- Fields, A.C.; Cotsonis, G.; Sexton, D.; Santoianni, R.; Cohen, C. Survivin expression in hepatocellular carcinoma: Correlation with proliferation, prognostic parameters, and outcome. Mod. Pathol. 2004, 17, 1378–1385. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, M.; Cao, M. Potential targets for molecular imaging of apoptosis resistance in hepatocellular carcinoma. Biomed. Imaging Interv. J. 2011, 7, e5. [Google Scholar] [PubMed]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic BAK is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Stacey, D.W. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr. Opin. Cell Biol. 2003, 15, 158–163. [Google Scholar] [CrossRef]
- Che, Y.; Ye, F.; Xu, R.; Qing, H.; Wang, X.; Yin, F.; Cui, M.; Burstein, D.; Jiang, B.; Zhang, D.Y. Co-expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am. J. Pathol. 2012, 180, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wu, R.H.; Wang, W.S. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-kappaB inactivation in SK-Hep1 cells. Oncol. Lett. 2017, 14, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Han, H.J.; Kim, W.B.; Song, T.J.; Choi, S.Y. VEGF Overexpression Predicts Poor Survival in Hepatocellular Carcinoma. Open Medicine 2017, 12, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Nart, D.; Yaman, B.; Yilmaz, F.; Zeytunlu, M.; Karasu, Z.; Kilic, M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transplant. 2010, 16, 621–630. [Google Scholar]
- Liu, Y.C.; Chiang, I.T.; Hsu, F.T.; Hwang, J.J. Using NF-kappaB as a molecular target for theranostics in radiation oncology research. Expert Rev. Mol. Diagn. 2012, 12, 139–146. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-kappaB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nature reviews. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Tsai, J.J.; Pan, P.J.; Hsu, F.T. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-kappaB activation in hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.T.; Liu, Y.C.; Wang, W.H.; Hsu, F.T.; Chen, H.W.; Lin, W.J.; Chang, W.Y.; Hwang, J.J. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-kappaB pathway in hepatocellular carcinoma cells. In Vivo 2012, 26, 671–681. [Google Scholar] [PubMed]
- Wu, J.Y.; Lin, S.S.; Hsu, F.T.; Chung, J.G. Fluoxetine Inhibits DNA Repair and NF-kB-modulated Metastatic Potential in Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.H.; Hsieh, C.L.; Liu, T.T.; Huang, C.S.; Chen, Y.C.; Chuang, Y.C.; Lin, S.S.; Hsu, F.T. Amentoflavone Induces Apoptosis and Inhibits NF-kB-modulated Anti-apoptotic Signaling in Glioblastoma Cells. In Vivo 2018, 32, 279–285. [Google Scholar] [PubMed]
- Li, L.; Zhao, G.D.; Shi, Z.; Qi, L.L.; Zhou, L.Y.; Fu, Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Wohlschlaeger, J.; Lang, H.; Sotiropoulos, G.C.; Malago, M.; Steveling, K.; Reis, H.; Cicinnati, V.R.; Schmid, K.W.; Baba, H.A. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J. Hepatol. 2008, 48, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.C.; Wang, M.H.; Tsai, J.J.; Kuo, Y.C.; Liu, Y.C.; Hsu, F.T.; Wang, H.E. Regorafenib inhibits tumor progression through suppression of ERK/NF-kappaB activation in hepatocellular carcinoma bearing mice. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lu, H.F.; Hsu, S.C.; Kuo, C.L.; Chang, S.J.; Lin, J.J.; Wu, P.P.; Liu, J.Y.; Lee, C.H.; Chung, J.G.; et al. Bufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-kB and matrix metalloproteinase-2/-9-signaling pathways. Environ. Toxicol. 2015, 30, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Tsai, J.J.; Tseng, C.W.; Kuo, Y.C.; Chuang, Y.C.; Lin, S.S.; Hsu, F.T. Amentoflavone Inhibits ERK-modulated Tumor Progression in Hepatocellular Carcinoma In Vitro. In Vivo 2018, 32, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.T.; Liu, Y.C.; Hsu, F.T.; Chien, Y.C.; Kao, C.H.; Lin, W.J.; Chung, J.G.; Hwang, J.J. Curcumin synergistically enhances the radiosensitivity of human oral squamous cell carcinoma via suppression of radiation-induced NF-kappaB activity. Oncol. Rep. 2014, 31, 1729–1737. [Google Scholar] [CrossRef]
- Su, J.; An, X.-R.; Li, Q.; Li, X.-X.; Cong, X.-D.; Xu, M. Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci. Rep. 2018, 8, 12620. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.T.; Sun, C.C.; Wu, C.H.; Lee, Y.J.; Chiang, C.H.; Wang, W.S. Regorafenib Induces Apoptosis and Inhibits Metastatic Potential of Human Bladder Carcinoma Cells. Anticancer Res. 2017, 37, 4919–4926. [Google Scholar] [PubMed]
- Wang, W.H.; Chiang, I.T.; Ding, K.; Chung, J.G.; Lin, W.J.; Lin, S.S.; Hwang, J.J. Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: Critical role of ca(+2)-dependent pathway. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 512907. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Shestakov, A.; Eriksson, K.; Chiodi, F. Role of Fas/FasL in regulation of inflammation in vaginal tissue during HSV-2 infection. Cell Death Disease 2011, 2, e132. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Wang, H.E.; Yu, C.C.; Liu, H.C.; Liu, Y.C.; Chiang, I.T. Curcumin Triggers DNA Damage and Inhibits Expression of DNA Repair Proteins in Human Lung Cancer Cells. Anticancer Res. 2015, 35, 3867–3873. [Google Scholar] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-T.; Hsu, F.-T.; Liu, Y.-C.; Chen, C.-H.; Hsu, L.-C.; Lin, S.-S. Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 757. https://doi.org/10.3390/ijms20030757
Chen W-T, Hsu F-T, Liu Y-C, Chen C-H, Hsu L-C, Lin S-S. Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro. International Journal of Molecular Sciences. 2019; 20(3):757. https://doi.org/10.3390/ijms20030757
Chicago/Turabian StyleChen, Wei-Ting, Fei-Ting Hsu, Yu-Chang Liu, Cheng-Hsien Chen, Li-Cho Hsu, and Song-Shei Lin. 2019. "Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro" International Journal of Molecular Sciences 20, no. 3: 757. https://doi.org/10.3390/ijms20030757