Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patient-Derived Melanoma Growing Orthotopically in Nude Mice
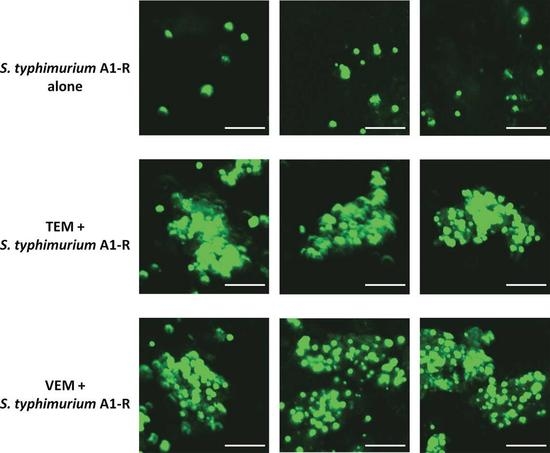
2.2. S. typhimurium A1-R Was Highly Effective on the Patient-Derived Orthotopic Xenograft (PDOX) Melanoma in Nude Mice
2.3. PDOX Model of a BRAF-V600E Mutant Melanoma
3. Materials and Methods
3.1. Mice
3.2. Patient-Derived Tumors
3.3. Establishment of PDOX Models of Melanoma by Surgical Orthotopic Implantation (SOI)
3.4. Preparation and Administration of S. typhimurium A1-R
3.5. Recombinant Methionase (rMETase) Production
3.6. Tumor Histology
3.7. Confocal Microscopy
3.8. Treatment Study Design in the PDOX Model of Melanoma
3.9. Intratumor l-Methionine Levels
Conflicts of Interest
References
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Gandidi, S.; Massi, D.; Mandala, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systemic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Einhorn, L.H.; Meyers, M.L.; Saxman, S.; Destro, A.N.; Panageas, K.S.; Begg, C.B.; Agarwala, S.S.; Schuchter, L.M.; Ernstoff, M.S.; et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J. Clin. Oncol. 1999, 17, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, Y.; Chlewicki, L.K.; Zhang, Y.; Guo, J.; Liang, W.; Wang, J.; Wang, X.; Fu, Y.X. Facilitating T Cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 2016, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Ascierto, P.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandal, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- McArthur, G.A.; Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Dummer, R.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; et al. Safety and efficacy of vemurafenib in BRAF (V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Zhao, M.; Zhang, Y.; Chmielowski, B.; Kiyuna, T.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Cell. Biochem. 2017, 118, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 2015, 15, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Guadagni, F.; Hoffman, R.M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 1992, 89, 5645–5649. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Murakami, T.; Momiyama, M.; Mori, R.; Matsuyama, R.; Katz, M.H.; Fleming, J.B.; Chishima, T.; et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLoS ONE 2014, 9, e114310. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Zhang, Y.; Murakami, T.; Maawy, A.A.; Miwa, S.; Yamamoto, M.; Yano, S.; Sato, S.; Momiyama, M.; Mori, R.; et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget 2014, 5, 12346–12357. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.A.; Katz, M.H.; Fleming, J.B.; Bouvet, M.; Endo, I.; Hoffman, R.M. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J. Surg. Oncol. 2015, 111, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Le, P.; Hoffman, R.M. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993, 13, 901–904. [Google Scholar] [PubMed]
- Fu, X.; Hoffman, R.M. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993, 13, 283–286. [Google Scholar] [PubMed]
- Wang, X.; Fu, X.; Hoffman, R.M. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int. J. Cancer 1992, 51, 992–995. [Google Scholar] [PubMed]
- Hiroshima, Y.; Zhang, Y.; Zhang, M.; Maawy, A.; Mii, S.; Yamamoto, M.; Uehara, F.; Miwa, S.; Yano, S.; Murakami, T.; et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS ONE 2015, 10, e0117417. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Besterman, J.M.; Monosov, A.; Hoffman, R.M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 1991, 88, 9345–9349. [Google Scholar] [CrossRef] [PubMed]
- Metildi, C.A.; Kaushal, S.; Luiken, G.A.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Surg. Oncol. 2014, 109, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.; Metildi, C.A.; Zhang, Y.; Uehara, F.; Miwa, S.; Yano, S.; Sato, S.; Murakami, T.; Momiyama, M.; et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J. Laparoendosc. Adv. Surg. Tech. 2014, 24, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Kubota, T.; Watanabe, M.; Kitajima, M.; Fu, X.; Hoffman, R.M. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int. J. Cancer 1993, 53, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Zhang, Y.; Zhang, N.; Uehara, F.; Maawy, A.; Murakami, T.; Mii, S.; Yamamoto, M.; Miwa, S.; Yano, S.; et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015, 35, 697–701. [Google Scholar] [PubMed]
- Hiroshima, Y.; Zhao, M.; Zhang, Y.; Zhang, N.; Maawy, A.; Murakami, T.; Mii, S.; Uehara, F.; Yamamoto, M.; Miwa, S.; et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS ONE 2015, 10, e0134324. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; DeLong, J.; Eilber, F.C.; Zhao, M.; Zhang, Y.; Zhang, N.; Singh, A.; Russell, T.; Deng, S.; Reynoso, J.; et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget 2016, 7, 12783–12790. [Google Scholar] [CrossRef] [PubMed]
- Kiyuna, T.; Murakami, T.; Tome, Y.; Kawaguchi, K.; Igarashi, K.; Zhang, Y.; Zhao, M.; Li, Y.; Bouvet, M.; Kanaya, F.; et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft nude mouse model. Oncotarget 2016, 7, 33046–33054. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Singh, A.S.; Kiyuna, T.; Dry, S.M.; Li, Y.; James, A.W.; Igarashi, K.; Kawaguchi, K.; DeLong, J.C.; Zhang, Y.; et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 2016, 7, 47556–47564. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.; Sato, S.; Murakami, T.; Uehara, F.; Miwa, S.; Yano, S.; Momiyama, M.; Chishima, T.; Tanaka, K.; et al. Hand-held high-resolution fluorescence imaging system for fluorescence-guided surgery of patient and cell-line pancreatic tumors growing orthotopically in nude mice. J. Surg. Res. 2014, 187, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Zhao, M.; Maawy, A.; Zhang, Y.; Katz, M.H.; Fleming, J.B.; Uehara, F.; Miwa, S.; Yano, S.; Momiyama, M.; et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J. Cell. Biochem. 2014, 115, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, A.; Katz, M.; Fleming, J.; Truty, M.; Thomas, R.; Saji, S.; Moriwaki, H.; Bouvet, M.; Hoffman, R.M. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res. 2012, 32, 3063–3067. [Google Scholar] [PubMed]
- Suetsugu, A.; Katz, M.; Fleming, J.; Truty, M.; Thomas, R.; Saji, S.; Moriwaki, H.; Bouvet, M.; Hoffman, R.M. Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with human-patient primary pancreatic cancer specimens. Anticancer Res. 2012, 32, 1175–1180. [Google Scholar] [PubMed]
- Yamamoto, M.; Zhao, M.; Hiroshima, Y.; Zhang, Y.; Shurell, E.; Eilber, F.C.; Bouvet, M.; Noda, M.; Hoffman, R.M. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS ONE 2016, 11, e0160882. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Zhao, M. Methods for the development of tumor-targeting bacteria. Expert Opin. Drug Discov. 2014, 9, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, M.; Li, X.M.; Jiang, P.; Baranov, E.; Li, S.; Xu, M.; Penman, S.; Hoffman, R.M. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2005, 102, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Geller, J.; Ma, H.; Yang, M.; Penman, S.; Hoffman, R.M. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 10170–10174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, M.; Ma, H.; Li, X.; Tan, X.; Li, S.; Yang, Z.; Hoffman, R.M. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006, 66, 7647–7652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tome, Y.; Suetsugu, A.; Zhang, L.; Zhang, N.; Hoffman, R.M.; Zhao, M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res. 2012, 32, 2501–2508. [Google Scholar] [PubMed]
- Zhang, Y.; Miwa, S.; Zhang, N.; Hoffman, R.M.; Zhao, M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015, 6, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Uchugonova, A.; Zhao, M.; Zhang, Y.; Weinigel, M.; König, K.; Hoffman, R.M. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012, 32, 4331–4339. [Google Scholar] [PubMed]
- Liu, F.; Zhang, L.; Hoffman, R.M.; Zhao, M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010, 9, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, C.; Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R.M. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009, 29, 1873–1878. [Google Scholar] [PubMed]
- Yam, C.; Zhao, M.; Hayashi, K.; Ma, H.; Kishimoto, H.; McElroy, M.; Bouvet, M.; Hoffman, R.M. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J. Surg. Res. 2010, 164, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Zhao, M.; Zhang, Y.; Maawy, A.; Hassanein, M.K.; Uehara, F.; Miwa, S.; Yano, S.; Momiyama, M.; Suetsugu, A.; et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013, 12, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Miwa, S.; Zhang, Y.; Hiroshima, Y.; Yano, S.; Uehara, F.; Yamamoto, M.; Toneri, M.; Bouvet, M.; Matsubara, H.; et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J. Cell. Biochem. 2014, 115, 1996–2003. [Google Scholar] [PubMed]
- Matsumoto, Y.; Miwa, S.; Zhang, Y.; Zhao, M.; Yano, S.; Uehara, F.; Yamamoto, M.; Hiroshima, Y.; Toneri, M.; Bouvet, M.; et al. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget 2015, 6, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Zhang, Y.; Zhao, M.; Hiroshima, Y.; Miwa, S.; Uehara, F.; Kishimoto, H.; Tazawa, H.; Bouvet, M.; Fujiwara, T.; et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014, 13, 3958–3963. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Zhang, Y.; Zhao, M.; Zhang, N.; Murakami, T.; Maawy, A.; Mii, S.; Uehara, F.; Yamamoto, M.; Miwa, S.; et al. Tumor-targeting Salmonella typhimurium A1-R in combination with Trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS ONE 2015, 10, e0120358. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Hoffman, R.M. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J. Cell. Biochem. 2009, 106, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Kishimoto, H.; Bouvet, M.; Hoffman, R.M. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle 2009, 8, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Zhang, Y.; Baek, K.-E.; Uehara, F.; Yano, S.; Yamamoto, M.; Hiroshima, Y.; Matsumoto, Y.; Kimura, H.; Hayashi, K.; et al. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget 2014, 5, 12849–12861. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Zhang, L.; Zhao, M.; Hayashi, K.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Wessels, J.; Hoffman, R.M. Targeted therapy of spinal cord glioma with a genetically-modified Salmonella typhimurium. Cell Prolif. 2010, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, M.; Zhao, M.; Kimura, H.; Tran, B.; Chishima, T.; Bouvet, M.; Endo, I.; Hoffman, R.M. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle 2012, 11, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Murakami, T.; Chmielowski, B.; Igarashi, K.; Kiyuna, T.; Unno, M.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; Li, Y.; et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2016, 7, 71737–71743. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Chmiewloski, B.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Singh, A.; Unno, M.; Nelson, S.D.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016, 7, 85929–85936. [Google Scholar] [PubMed]
- Kawaguchi, K.; Igarashi, K.; Chmielowski, B.; Murakami, T.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; et al. Salmonella typhimurium A1-R targeting of a chemotherapy resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozlomide. Cell Cycle 2017, 16, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lishko, V.K.; Herrera, H.; Groce, A.; Kubota, T.; Hoffman, R.M. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. 1993, 53, 5676–5679. [Google Scholar] [PubMed]
- Hoffman, R.M.; Jacobsen, S.J. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc. Natl. Acad. Sci. USA 1980, 77, 7306–7710. [Google Scholar] [CrossRef] [PubMed]
- Kokkinakis, D.M.; von Wronski, M.A.; Vuong, T.H.; Brent, T.P.; Schold, S.C., Jr. Regulation of O6-methylguanine-DNA methyltransferase by methionine in human tumour cells. Br. J. Cancer 1997, 75, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Kokkinakis, D.M.; Schold, S.C., Jr.; Hori, H.; Nobori, T. Effect of long-term depletion of plasma methionine on the growth and survival of human brain tumor xenografts in athymic mice. Nutr. Cancer 1997, 29, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Erbe, R.W. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc. Natl. Acad. Sci. USA 1976, 73, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.H.; Mecham, J.O.; Wallace, C.D.; Hoffman, R.M. Reduced free-methionine in methionine-dependent SV40-transformed human fibroblasts synthesizing apparently normal amounts of methionine. J. Cell. Physiol. 1983, 117, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.H.; Wallace, C.D.; Hoffman, R.M. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J. Cell. Physiol. 1984, 119, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.H.; Hoffman, R.M. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro 1984, 20, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis: A review and synthesis. Biochim. Biophys. Acta 1984, 738, 49–87. [Google Scholar] [CrossRef]
- Coalson, D.W.; Mecham, J.O.; Stern, P.H.; Hoffman, R.M. Reduced availability of endogenously synthesized methionine for S-adenosylmethionine formation in methionine dependent cancer cells. Proc. Natl. Acad. Sci. USA 1982, 79, 4248–4251. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Is DNA methylation the new guardian of genome? Mol. Cytogenet. 2017, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. The wayward methyl group and the cascade to cancer. Cell Cycle 2017, 16, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Lishko, V.K.; Lishko, O.V.; Hoffman, R.M. The preparation of endotoxin-free l-methionine-α-deamino-γ-mercaptomethane-lyase (l-methioninase) from Pseudomonas putida. Protein Expr. Purif. 1993, 4, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lishko, V.K.; Lishko, O.V.; Hoffman, R.M. Depletion of serum methionine by methioninase in mice. Anticancer Res. 1993, 13, 1465–1468. [Google Scholar] [PubMed]
- Tan, Y.; Zavala, J., Sr.; Xu, M.; Zavala, J., Jr.; Hoffman, R.M. Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res. 1996, 16, 3937–3942. [Google Scholar] [PubMed]
- Tan, Y.; Zavala, J., Sr.; Han, Q.; Xu, M.; Sun, X.; Tan, X.; Tan, X.; Magana, R.; Geller, J.; Hoffman, R.M. Recombinant methioninase infusion reduces the biochemical endpoint of serum methionine with minimal toxicity in high-stage cancer patients. Anticancer Res. 1997, 17, 3857–3860. [Google Scholar] [PubMed]
- Tan, Y.; Xu, M.; Tan, X.; Tan, X.; Wang, X.; Saikawa, Y.; Nagahama, T.; Sun, X.; Lenz, M.; Hoffman, R.M. Overexpression and large-scale production of recombinant l-methionine-α-deamino-γ-mercaptomethane-lyase for novel anticancer therapy. Protein Expr. Purif. 1997, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Inagaki, K.; Sugimoto, M.; Esaki, N.; Soda, K.; Tanaka, H. Structural analysis of the l-methionine γ-lyase gene from Pseudomonas putida. J. Biochem. 1995, 117, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Takabayashi, K.; Orvis, L.; Carson, D.A.; Nobori, T. Gene cloning and characterization of Pseudomonas putida l-methionine-α-deamino-γ-mercaptomethane-lyase. Cancer Res. 1996, 56, 2116–2122. [Google Scholar] [PubMed]
- Kawaguchi, K.; Igarashi, K.; Li, S.; Han, Q.; Tan, Y.; Kiyuna, T.; Miyake, Y.; Murakami, T.; Chmielowski, B.; Nelson, S.D.; et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma growth in a patient-derived orthotopic xenograft. Oncotarget 2017. [Google Scholar] [CrossRef]
- Pimiento, J.M.; Larkin, E.M.; Smalley, K.S.; Wiersma, G.L.; Monks, N.R.; Fedorenko, I.V.; Peterson, C.A.; Nickoloff, B.J. Melanoma genotypes and phenotypes get personal. Lab Investig. 2013, 93, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L.; Joseph, R.W.; Copland, J.A. Patient-derived tumor xenograft models for melanoma drug discovery. Expert Opin. Drug Discov. 2016, 11, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Uchugonova, A.; Duong, J.; Zhang, N.; König, K.; Hoffman, R.M. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J. Cell. Biochem. 2011, 112, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Li, S.; Han, Q.; Tan, Y.; Kiyuna, T.; Igarashi, K.; Kawaguchi, K.; Hwang, H.K.; Miyaki, K.; Singh, A.S.; et al. Recombinant methioninase effectively targets a Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 2017, 8, 35630–35638. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, Y.; Yang, Z.; Li, S.; Hoffman, R.M. A rapid HPLC method for the measurement of ultra-low plasma methionine concentrations applicable to methionine depletion therapy. Anticancer Res. 2005, 25, 59–62. [Google Scholar] [PubMed]










© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, R.M. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. Int. J. Mol. Sci. 2017, 18, 1875. https://doi.org/10.3390/ijms18091875
Hoffman RM. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. International Journal of Molecular Sciences. 2017; 18(9):1875. https://doi.org/10.3390/ijms18091875
Chicago/Turabian StyleHoffman, Robert M. 2017. "Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma" International Journal of Molecular Sciences 18, no. 9: 1875. https://doi.org/10.3390/ijms18091875