- Department of Neurosurgery, Cannizzaro Hospital, Via Messina 829, Catania,
- Department of Neurosurgery, Hospital “San Gerardo,” Via G. B. Pergolesi 33, Monza, Italy,
- Department of Amethyst Radiotherapy Center, San Giovanni Calibita Fatebenefratelli Hospital, Tiber Island, Rome,
- Neurosurgery Unit, Highly Specialized Hospital and of National Importance “Garibaldi”, Piazza Santa Maria di Gesù 5, Catania, Italy.
Correspondence Address:
Giuseppe Emmanuele Umana
Neurosurgery Unit, Highly Specialized Hospital and of National Importance “Garibaldi”, Piazza Santa Maria di Gesù 5, Catania, Italy.
DOI:10.25259/SNI_145_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giuseppe Emmanuele Umana1, Giuseppe Raudino2, Nicola Alberio1, Francesco Inserra1, Giuseppe Giovinazzo3, Marco Fricia1, Stefano Chiriatti1, Giovanni Federico Nicoletti4, Salvatore Cicero1, Gianluca Scalia4. Slit-like hypertensive hydrocephalus: Report of a late, complex, and multifactorial complication in an oncologic patient. 01-Aug-2020;11:219
How to cite this URL: Giuseppe Emmanuele Umana1, Giuseppe Raudino2, Nicola Alberio1, Francesco Inserra1, Giuseppe Giovinazzo3, Marco Fricia1, Stefano Chiriatti1, Giovanni Federico Nicoletti4, Salvatore Cicero1, Gianluca Scalia4. Slit-like hypertensive hydrocephalus: Report of a late, complex, and multifactorial complication in an oncologic patient. 01-Aug-2020;11:219. Available from: https://surgicalneurologyint.com/surgicalint-articles/10173/
Abstract
Background: Several sophisticated techniques and many chemotherapy drugs have improved life expectancy of oncologic patients allowing us to observe late complications which present many years after the initial treatment.
Case Description: We present a unique case of a patient affected by acute lymphoblastic leukemia at the age of 6 years, treated with whole brain radiotherapy and intrathecal chemotherapy, developing meningiomatosis and leptomeningeal alterations as late complications and the interaction of these two entities caused a peculiar form of hydrocephalus without ventricular dilation. The diagnosis of pseudotumor cerebri was excluded due the postradio/chemotherapy development of meningiomatosis, not present in a previously head magnetic resonance imaging, that exerted compression to the Sylvian aqueduct causing intracranial hypertension with papillary stasis without ventricles enlargement due to brain stiffness. Moreover, a peculiar intraoperative rubbery consistency of brain parenchyma was detected strengthening this complex diagnosis.
Conclusion: At the best of our knowledge, this is the first report of obstructive hydrocephalus without ventricles dilation caused by brain stiffness related to late alterations of oncologic treatments. This report could be a guide for further complex patients diagnoses and for improving treatments efficacy.
Keywords: Hydrocephalus, Intrathecal chemotherapy, Meningiomatosis, Radiotherapy, Slit ventricle
INTRODUCTION
Life expectancy of oncologic patients, both adults and pediatric, has progressively improved with the development of modern treatments and diagnostics. Several sophisticated techniques and many chemotherapy drugs fit with the single patient’s needs. A positive consequence of these improvements is the emergence of long-term survivors. This has led to very long follow-ups that can teach us important lessons, as now we have the chance to observe late complications which present many years after the initial treatment.
We present a unique case of a patient affected by acute lymphoblastic leukemia at the age of 6 years, treated with whole brain radiotherapy (WBRT) and intrathecal chemotherapy, who developed cerebral meningiomatosis and leptomeningeal alterations as late complications, and the interaction of these two entities caused a peculiar form of hydrocephalus without ventricular dilation.
CASE DESCRIPTION
Clinical presentation
A 35-year-old female was admitted to our institution because she had been suffering from progressive headaches for about 10 years – referred as debilitating in the past 6 months – which worsen with emotions like rage or concern, associated with blurred vision and tinnitus. Mini-mental state examination (MMSE) gave a score of 23.
Medical history
Her clinical history was positive for acute lymphoblastic leukemia, diagnosed in 1990 and treated with AIEOP 8703 protocol (cobalt radiotherapy [RT] in pediatric age and intrathecal chemotherapy with IV vincristine, IV daunorubicin, prednisone, methotrexate intrathecal, and IM L-asparaginase). In 1995, she was declared in complete remission. A right oophorectomy at the age of 9, caused by ovarian torsion, was also reported. In 2000, she underwent brain magnetic resonance imaging (MRI) that did not find pathological alterations. In 2011, she underwent in vitro fertilization, which unfortunately failed.
Preoperative imaging and instrumental assessment
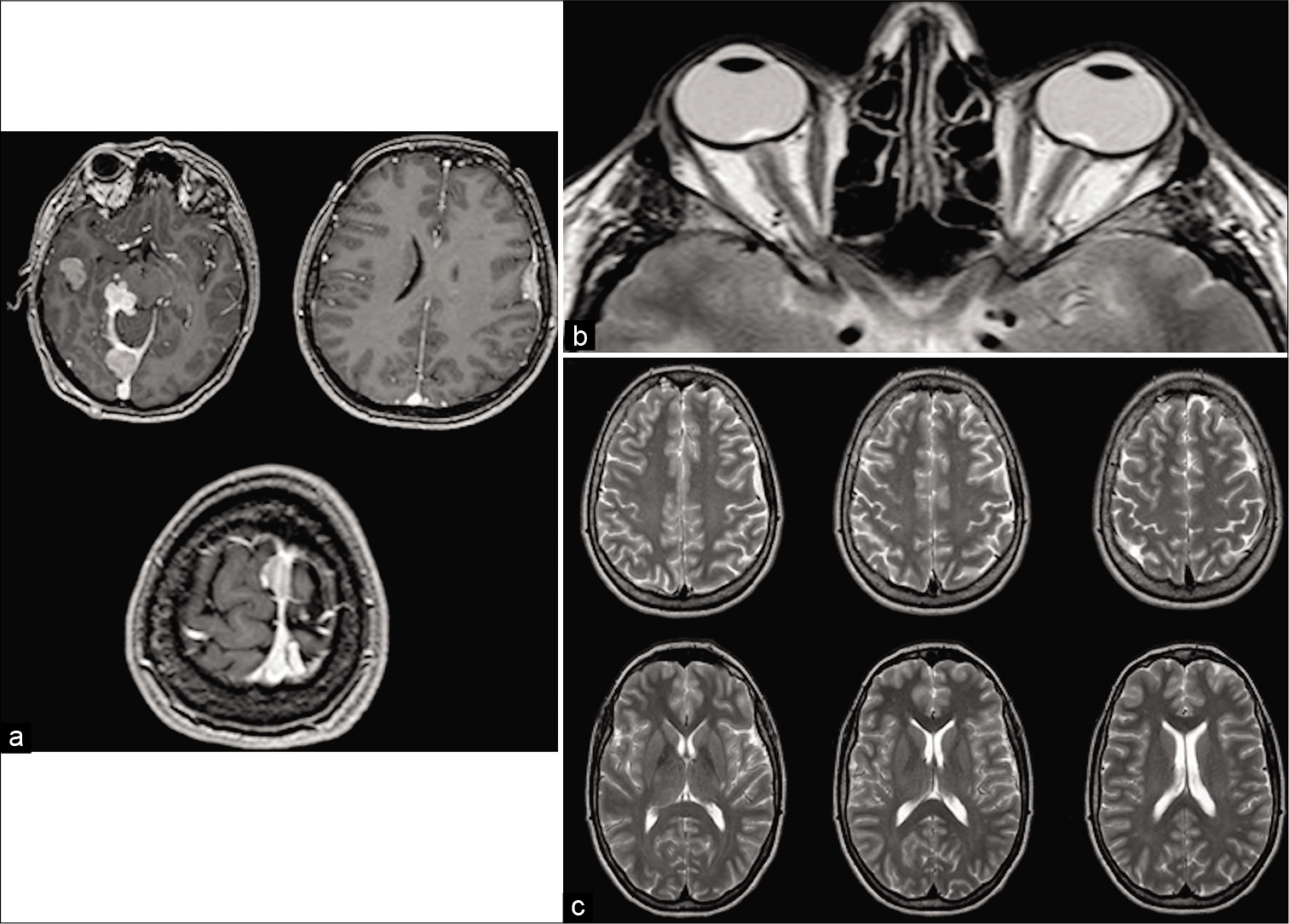
The patient underwent in-depth investigation that documented cerebral meningiomatosis, characterized by 5 meningiomas, <3 cm each, located at the right cerebral middle fossa, at the border of the right tentorium, at the torcula, at the middle third of the superior sagittal sinus (SSS), and at the left Sylvian fissure; a T2-weighted brain MRI sequence documented a typical bilateral enlargement of optic nerve sheath, related to intracranial hypertension; of notice, ventricles were not enlarged, but they presented slit-like aspect [
Figure 1:
Gadolinium T1-weighted magnetic resonance imaging images showing postcontrast enhancement of five meningiomas, <3 cm each, located at the right cerebral middle fossa, at the border of the right tentorium, at the torcula, at the middle third of the superior sagittal sinus , and at the left Sylvian fissure (a). T2-weighted sequences documented typical bilateral enlargement of optic nerve sheath, related to intracranial hypertension; of notice, ventricles were not enlarged, conversely, they present slit-like aspect (b). T2-weighted Turbo spin echo brain magnetic resonance imaging sequences showing normal representation of cisterns and sulci that rule out the hypothesis of pseudotumor cerebri (c).
Clinical considerations and management
In our opinion, the patient was not affected by pseudotumor cerebri due to the normal global brain anatomy with good representation of the cisterns and sulci and the very high ICP values. Our hypothesis was that the absence of hydrocephalus was related to previous RT and intrathecal chemotherapy in pediatric age and that the tentorial meningioma causes compression of the Sylvian aqueduct; we elaborated two options for a multimodal treatment strategy: (a) surgical resection of the tentorial meningioma and Gamma Knife treatment to control the other lesions, with ventriculoperitoneal shunt (VPS) to be considered in a second surgical time after clinical postoperative evaluation and (b) VPS to resolve intracranial hypertension and radiosurgery to control mainly the tentorial meningioma and avoid further growth with associated compression of the brainstem. In agreement with the patient, we decided to perform the latter option. First, she underwent Gamma Knife treatment of the tentorial meningioma because the presence of the ventricular catheter could cause artifacts and negatively affect the accuracy of radiosurgery planning. The following day, with the guidance of the neuronavigation, we introduced the ventricular catheter at the right Kocher’s point and connected the distal catheter with a single route, without the typical skin interruption at the cervical level, to the peritoneum in the epigastric-suprahepatic site. A programmable valve with virtual off option was implanted and set at the 5th setting (145 mmH2O).
Postoperative imaging and clinical follow-up
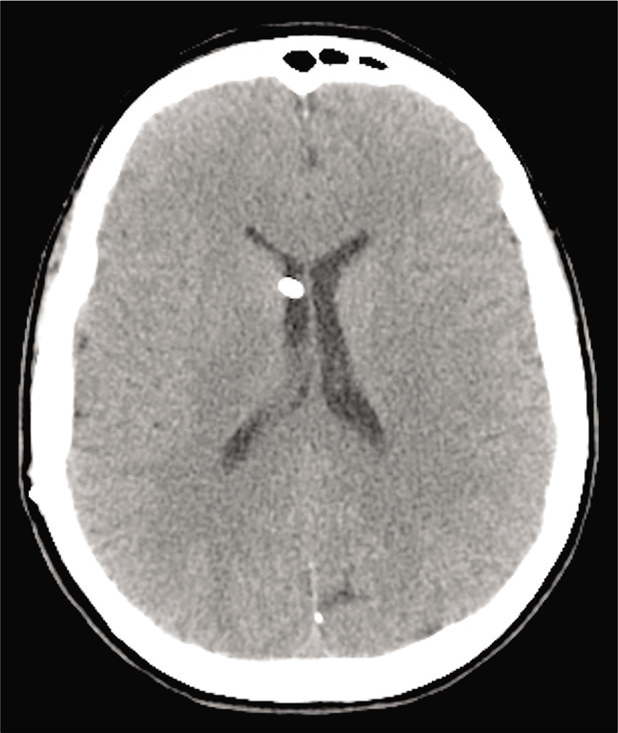
The postoperative period was uneventful, a postoperative brain computed tomography scan [
After a 6-month follow-up, she is doing well, without clinical disturbance. MMSE gave a score of 25. She will continue to be followed for the other meningiomas and the VPS function with brain MRI every year and further Gamma Knife treatment of the lesions.
DISCUSSION
It is known that RT in children affected by cancer can cause structural changes in the brain, linked to cognitive late effects [
The aim of intrathecal chemotherapy is to increase central nervous system drug exposure through direct CSF introduction, while reducing systemic drug toxicity.[
Intrathecal chemotherapy can cause potentially tragic effects. Chemical arachnoiditis, characterized by headache, back pain, vomiting, fever, meningism, and CSF pleocytosis, is common and potentially serious consequences.[
Gállego Pérez-Larraya et al.[
CONCLUSION
With the present report, we suggest an in-depth and complex diagnosis to justify a very rare case characterized by concomitant pathologies, multifactorial direct and indirect effects of the disease, and late radio/chemotherapy side effects. At the best of our knowledge, this is the first report of obstructive hydrocephalus without ventricles dilation caused by brain stiffness related to oncologic treatments. To achieve these complex diagnostic conclusions, we conducted a multidisciplinary and multicentric debate. This report could be a guide for further complex patients diagnoses and for improving treatments efficacy. MRE could represent a useful tool, if available, but further implementation and validation of the technique are required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the childhood cancer survivor study. J Natl Cancer Inst. 2009. 101: 946-58
2. Atkinson S, Li YQ, Wong CS. Changes in oligodendrocytes and myelin gene expression after radiation in the rodent spinal cord. Int J Radiat Oncol Biol Phys. 2003. 57: 1093-100
3. Beera KG, Li YQ, Dazai J, Stewart J, Egan S, Ahmed M. Altered brain morphology after focal radiation reveals impact of off-target effects: Implications for white matter development and neurogenesis. Neuro Oncol. 2018. 20: 788-98
4. Bertalan G, Klein C, Schreyer S, Steiner B, Kreft B, Tzschätzsch H. Biomechanical properties of the hypoxic and dying brain quantified by magnetic resonance elastography. Acta Biomater. 2020. 101: 395-402
5. Bleyer WA. The clinical pharmacology of methotrexate: New applications of an old drug. Cancer. 1978. 41: 36-51
6. Boström M, Kalm M, Karlsson N, Erkenstam NH, Blomgren K. Irradiation to the young mouse brain caused long-term, progressive de pletion of neurogenesis but did not disrupt the neurovascular niche. J Cereb Blood Flow Metab. 2013. 33: 935-43
7. Byrnes DM, Vargas F, Dermarkarian C, Kahn R, Kwon D, Hurley J. Complications of intrathecal chemotherapy in adults: Single-institution experience in 109 consecutive patients. J Oncol. 2019. 2019: 4047617
8. Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol. 1996. 29: 91-101
9. Gàllego Pérez-Larraya JG, Palma JA, Carmona-Iragui M, Fernández-Torrón R, Irimia P, Rodríguez-Otero P. Neurologic complications of intrathecal liposomal cytarabine administered prophylactically to patients with non-Hodgkin lymphoma. J Neurooncol. 2011. 103: 603-9
10. Kalra P, Raterman B, Mo X, Kolipaka A. Magnetic resonance elastography of brain: Comparison between anisotropic and isotropic stiffness and its correlation to age. Magn Reson Med. 2019. 82: 671-9
11. Kerr JZ, Berg S, Blaney SM. Intrathecal chemotherapy. Crit Rev Oncol Hematol. 2001. 37: 227-36
12. Kurt M, Wu L, Laksari K, Ozkaya E, Suar ZM, Lv H. Optimization of a multifrequency magnetic resonance elastography protocol for the human brain. J Neuroimaging. 2019. 29: 440-6
13. Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: Drugs and toxicities. Ann Hematol. 2009. 88: 193-201
14. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002. 8: 955-62
15. Nieman BJ, de Guzman AE, Gazdzinski LM, Chakravarty MM, Pipitone J, Strother D. White and gray matter abnormalities after cranial radiation in children and mice. Int J Radiat Oncol Biol Phys. 2015. 93: 882-91
16. Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: Longitudinal MR imaging study. AJNR Am J Neuroradiol. 2002. 23: 1088-94
17. Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci USA. 2013. 110: 12822-7
18. Reddick WE, Glass JO, Palmer SL, Wu S, Gajjar A, Langston JW. Atypical white matter volume development in children following craniospinal irradiation. Neuro Oncol. 2005. 7: 12-9
19. Resar LM, Phillips PC, Kastan MB, Leventhal BG, Bowman PW, Civin CI. Acute neurotoxicity afer intrathecal cytosine arabinoside in two adolescents with acute lymphoblastic leukemia of B-cell type. Cancer. 1993. 71: 117-23
20. Riggs L, Bouffet E, Laughlin S, Laperriere N, Liu F, Skocic J. Changes to memory structures in children treated for posterior fossa tumors. J Int Neuropsychol Soc. 2014. 20: 168-80
21. Takamura T, Motosugi U, Sasaki Y, Kakegawa T, Sato K, Glaser KJ. Influence of age on global and regional brain stiffness in young and middle-aged adults. J Magn Reson Imaging. 2019. 51: 727-33