Summary
Abstract
Bevacizumab (Avastin®) is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is used to inhibit VEGF function in vascular endothelial cells and thereby inhibit tumour angiogenesis, upon which solid tumours depend for growth and metastasis.
The addition of bevacizumab to fluoropyrimidine-based chemotherapy, with or without irinotecan or oxaliplatin, in both the first- and second-line treatment of metastatic colorectal cancer, significantly increased median progression-free survival or time to disease progression in most randomized controlled trials. Bevacizumab was generally, but not always, associated with a survival advantage; in phase III trials, the increases in median overall survival attributable to bevacizumab were 4.7 months with first-line therapy and 2.1 months with second-line therapy. In some studies, patients experienced clinical improvement without an apparent overall survival benefit. Bevacizumab had acceptable tolerability, with the majority of adverse events being generally mild and clinically manageable. However, from the UK National Health Service perspective, bevacizumab was not considered to be cost effective in combination with bolus fluorouracil/folinic acid or irinotecan/bolus fluorouracil/folinic acid. Additional pharmaco-economic analyses from different perspectives and using clinical data for combinations with the more efficacious infusional fluorouracil/folinic acid plus oxaliplatin or irinotecan chemotherapy regimens are required. Although cost effectiveness may be a concern, the combination of bevacizumab and fluoropyrimidine-based chemotherapy has potential in the treatment of metastatic colorectal cancer.
Pharmacological Properties
Bevacizumab, produced by incorporating six VEGF-binding residues from a murine anti-human VEGF monoclonal antibody into a human IgG framework, binds to soluble VEGF and prevents it from binding to its receptors, VEGFR-1 or VEGFR-2, predominantly on vascular endothelial cells. VEGF activates and promotes the growth of vascular endothelial cells and is a potent regulator of angiogenesis and vascular permeability.
Bevacizumab and murine anti-VEGF monoclonal antibody had no effect on the growth of tumour cell lines in vitro, indicating that they do not act directly on tumour cells. Treatment of mice bearing human colorectal cancer cell lines with bevacizumab or murine anti-VEGF monoclonal antibody produced dose-dependent reductions in tumour volume, reduced tumour blood vessel density, vascular permeability and liver metastases, and paradoxically increased tumour perfusion; resulting in increased intratumoural concentrations of irinotecan. Bevacizumab similarly reduced tumour volume and normalized tumour vasculature in patients with colorectal carcinoma, but in humans, tumour perfusion was reduced.
The pharmacokinetic profile of intravenous bevacizumab in patients with metastatic colorectal cancer was linear over the dose range 0.3–10 mg/kg, with some accumulation after multiple-dose administration of 10 mg/kg every 2 weeks. Steady-state levels of the drug were achieved after 100 days. The clearance of bevacizumab was low and varied with bodyweight, gender and tumour burden. Both the volume of distribution in the central compartment and clearance were higher in male than in female patients with metastatic colorectal cancer after correcting for bodyweight. The elimination half-life of bevacizumab is long (12–22 days), allowing administration once every 2–3 weeks. Bevacizumab did not affect, to any clinically relevant extent, the pharmacokinetics of common chemotherapy agents with which it might be administered.
Therapeutic Efficacy
In randomized controlled trials, the addition of intravenous bevacizumab to chemotherapy with bolus fluorouracil/folinic acid (FU/FA), irinotecan/bolus fluorouracil/folinic acid (IFL), irinotecan/infusional fluorouracil/folinic acid (FOLFIRI), infusional fluorouracil/folinic acid/oxaliplatin (FOLFOX), capecitabine/oxaliplatin (XELOX) or bolus fluorouracil/folinic acid/oxaliplatin generally displayed efficacy in the first-line treatment of metastatic colorectal cancer.
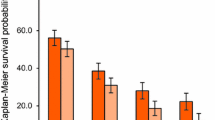
In a pivotal phase III trial, adding bevacizumab to IFL significantly improved overall survival, progression-free survival, objective tumour response rate and 1-year survival compared with IFL plus placebo. Similarly, a large phase III trial demonstrated that bevacizumab in combination with FOLFOX4 or XELOX significantly increased progression-free survival compared with FOLFOX4 or XELOX plus placebo, although bevacizumab had no effect on the objective response rate. Other controlled trials have generally demonstrated significantly improved progression-free survival, time to disease progression or response rates with the addition of bevacizumab compared with chemotherapy alone.
The addition of bevacizumab to FOLFOX4 for the second-line treatment of metastatic colorectal cancer also significantly improved overall survival, progression-free survival and the objective response rate compared with FOLFOX4 alone in a large phase III trial.
Controlled studies suggested that combining bevacizumab with cetuximab was practical and beneficial, but that combination with either panitumumab or erlotinib/XELOX was impractical due to reduced efficacy or an increase in severe adverse events.
Tolerability
The tolerability of bevacizumab was generally acceptable in patients with metastatic colorectal cancer. Adverse events were common in controlled clinical trials with bevacizumab, but most were of mild or moderate severity and many were related to the concomitant chemotherapy. Haemorrhage (mostly epistaxis), hypertension and proteinuria were relatively common bevacizumab-related adverse events, but were seldom of grade 3 (severe) or 4 (life-threatening) severity and were generally clinically manageable. In placebo-controlled trials of first-line therapy, only hypertension and any grade 3/4 event occurred with a significantly higher incidence in bevacizumab recipients than in placebo recipients. Arterial thrombotic events, gastrointestinal perforation and wound-healing complications were infrequent, but were potentially serious events and occasionally fatal.
Pharmacoeconomic Considerations
A cost-effectiveness analysis performed from the perspective of the UK National Health Service, using data from controlled clinical trials in which bevacizumab was added to bolus FU/FA or IFL, found that the incremental cost of bevacizumab per quality-adjusted life-year gained was £62 857 when added to IFL and £88 436 in combination with FU/FA; at least 2-fold higher than the generally accepted willingness-to-pay threshold of £30 000.
Similar content being viewed by others
References
International Agency for Research on Cancer. GLOBOCAN 2002 database [online]. Available from URL: http://wwwdep.iarc.fr [Accessed 2007 Dec 12]
Hoff PM. Bevacizumab in older patients and patients with poorer performance status. Semin Oncol 2006 Oct; 33 (5 Suppl. 10): S19–25
Natarajan N, Shuster TD. New agents, combinations, and opportunities in the treatment of advanced and early-stage colon cancer. Surg Clin North Am 2006 Aug; 86(4): 1023–43
Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 1992 Jun; 10(6): 896–903
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2000 Sep 28; 343(13): 905–14
de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000 Aug; 18(16): 2938–47
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004 Jan 15; 22(2): 229–37
Board RE, Valle JW. Metastatic colorectal cancer: current systemic treatment options. Drugs 2007; 67(13): 1851–67
Diaz-Rubio E. Vascular endothelial growth factor inhibitors in colon cancer. Adv Exp Med Biol 2006; 587: 251–75
Genentech Inc. Avastin® (bevacizumab) for intravenous use: US prescribing information [online]. Available from URL: http://www.gene.com/gene/products/information/oncology/avastin/insert.jsp [Accessed 2007 Dec 3]
European Medicines Agency. Avastin (bevacizumab) 25 mg/mL: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/avastin/H-582-PI-en.pdf [Accessed 2007 Jan 23]
Lyseng-Williamson KA, Robinson DM. Bevacizumab: a review of its use in advanced colorectal cancer, breast cancer, and NSCLC. Am J Cancer 2006; 5(1): 43–60
Scott LJ. Bevacizumab: in first-line treatment of metastatic breast cancer. Drugs 2007; 67(12): 1793–9
Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997 Oct 15; 57(20): 4593–9
Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004 Aug; 25(4): 581–611
André T, Kotelevets L, Vaillant JC, et al. VEGF, VEGF-B, VEGF-C and their receptors KDR, FLT-1 and FLT-4 during the neoplastic progression of human colonic mucosa. Int J Cancer 2000 Apr 15; 86(2): 174–81
Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993 Apr 29; 362: 841–4
Warren RS, Yuan H, Matli MR, et al. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995 Apr; 95: 1789–97
Yuan F, Chien Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A 1996 Dec; 93: 14765–70
Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 2003; 88: 1979–86
Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004 Feb; 10(2): 145–7
Koukourakis MI, Mavanis I, Kouklakis G, et al. Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol 2007 Jun; 30(3): 315–8
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004 Jun 3; 350(23): 2335–42
Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006 Jan 10; 24(2): 217–27
Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol 2001 Feb 1; 19(3): 851–6
Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005 Apr 10; 23(11): 2544–55
Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 2001 Feb 1; 19(3): 843–50
Gaudreault J, Lieberman G, Kabbinavar F, et al. Pharmaco-kinetics (PK) of bevacizumab (BV) in colorectal cancer (CRC) [abstract no. PI-98]. Clin Pharmacol Ther 2001 Feb; 69(2): P25
Brennan B, Siu L, Dhesy-Thind B, et al. Pharmacokinetic interactions between capecitabine, oxaliplatin and bevacizumab when used in combination for first-line treatment of metastatic colorectal cancer [abstract no. 2554]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 110s. Plus poster presented at the 43rd Annual Meeting of the American Society of Clinical Oncology; 2007 Jun 1–5; Chicago (IL)
Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003 Jan 1; 21(1): 60–5
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005 Jun 1; 23(16): 3697–705
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007 Apr 20; 25(12): 1539–44
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with XELOX or FOLFOX4: efficacy results from XELOX-1/NO16966, a randomized phase III trial in the first-line treatment of metastatic colorectal cancer [abstract no. 238, plus oral presentation]. 2007 Gastrointestinal Cancers Symposium; 2007 Jan 19–21; Orlando (FL)
Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: final analysis of the TREE-study [abstract no. 3510]. J Clin Oncol 2006 Jun 20; 24 (18 Suppl.): 148s. Plus oral presentation presented at the 42nd Annual Meeting of the American Society of Clinical Oncology; 2006 Jun 2–6; Atlanta (GA)
Tournigand C, Lledo G, Delord J, et al. Modified FOLFOX7/bevacizumab or modified XELOX/bevacizumab with or without erlotinib in first-line metastatic colorectal cancer: results of the feasibility phase of the DREAM-OPTIMOX3 study (GERCOR) [abstract no. 4097]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 187s
Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007 Oct 20; 25(30): 4779–86
Saltz LB, Lenz H-J, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 2007 Oct 10; 25(29): 4557–61
Hecht JR. Updates in the management of gastrointestinal cancers: a report from the 9th World Congress on Gastrointestinal Cancer (WCGIC) [online]. Available from URL: http://www.ufscc.ufl.edu/Professional/content.aspx?section=conferencecoverage&id=40270 [Accessed 2007 Nov 12]
Kozloff M, Hainsworth J, Badarinath S, et al. Efficacy of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE) [abstract no. 3537].J Clin Oncol 2006 Jun 20; 24 (18 Suppl.): 155s
Berry S, Cunningham D, Michael M, et al. Preliminary efficacy of bevacizumab with first-line FOLFOX, XELOX, FOLFIRI and fluoropyrimidines for mCRC: First BEAT trial. First BEAT Investigators [abstract no. 3020]. EJC Supplements 2007 Sep; 5(4): 241. Plus poster presented at the 14th European Cancer Conference; 2007 Sep 23–27; Barcelona
Saltz L, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with XELOX or FOLFOX4: updated efficacy results from XELOX-1/N016966, a randomized phase III trial in first-line metastatic colorectal cancer [abstract no. 4028]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 170s. Plus abstract presented at the 43rd Annual Meeting of the American Society of Clinical Oncology; 2007 Jun 1–5; Chicago (IL)
Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005 May 20; 23(15): 3502–8
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004 Jan 1; 22(1): 23–30
Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 2005 Aug 1; 23(22): 4866–75
Tyagi P, Grothey A. Commentary on a phase III trial of bevacizumab plus XELOX or FOLFOX4 for first-line treatment of metastatic colorectal cancer: the NO16966 trial. Clin Colorectal Cancer 2006 Nov; 6(4): 261–4
Cassidy J, Clarke S, Diaz-Rubio E, et al. XELOX compared to FOLFOX4: survival and response results from XELOX-1/N016966, a randomized phase III trial of first-line treatment for patients with metastatic colorectal cancer [abstract no. 4030]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 171s. Plus poster presented at the 43rd Annual Meeting of the American Society of Clinical Oncology; 2007 Jun 1–5; Chicago (IL)
Amgen. Amgen discontinues Vectibix(TM) treatment in PACCE trial evaluating Vectibix(TM) as part of triple combination regimen: preliminary pre-planned interim analysis shows negative effect on progression-free survival [media release]. 2007 Mar 22
Golfinopoulos V, Salanti G, Pavlidis N, et al. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol 2007 Oct; 8(10): 898–911
Kozloff M, Hainsworth J, Badarinath S, et al. Survival of patients (pts) with mCRC treated with bevacizumab in combination with chemotherapy: results from the BRiTE registry [abstract no. 375 plus poster]. 4th Annual Gastrointestinal Cancers Symposium; 2007 Jan 19–21; Orlando (FL)
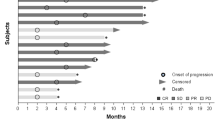
Grothey A, Sugrue M, Hedrick E, et al. Association between exposure to bevacizumab beyond first progression and overall survival in patients with metastatic colorectal cancer: results from a large observational study (BRiTE) [abstract no. 4036]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 172s. Plus poster presented at the 43rd Annual Meeting of the American Society of Clinical Oncology; 2007 Jun 1–5; Chicago (IL)
Hedrick E, Kozloff M, Hainsworth J, et al. Safety of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE) [abstract no. 3536]. J Clin Oncol 2006 Jun 20; 24 (18 Suppl.): 155s
Tappenden P, Jones R, Paisley S, et al. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer 2007 Nov; 43(17): 2487–94
National Institute for Health and Clinical Excellence. Bevacizumab and cetuximab for the treatment of metastatic colorectal cancer [online]. Available from URL: http://www.nice.org.uk/guidance/TA118/niceguidance/pdf/english [Accessed 2007 Oct 23]
Roche. Avastin receives broad label extension in Europe for the treatment of patients with metastatic colorectal cancer [media release]. 2008 Jan 28
de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997 Feb; 15(2): 808–15
Van Cutsem EJD. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. ESMO Guidelines Working Group. Ann Oncol 2007 Apr; 18 Suppl. 2: ii25–6
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. Version 1.2008 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf [Accessed 2007 Dec 17]
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer. Version 1.2008 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf [Accessed 2007 Dec 17]
O'Dwyer PJ. The present and future of angiogenesis-directed treatments of colorectal cancer. Oncologist 2006 Oct; 11(9): 992–8
Giantonio BJ. Bevacizumab in the treatment of metastatic colorectal cancer (mCRC) in second- and third-line settings. Semin Oncol 2006 Oct; 33 (5 Suppl. 10): S15–8
Samalin E, Liévre A, Boyer-Gestin C, et al. Efficacy of bevacizumab in combination with irinotecan or oxaliplatin as 2nd and 3rd or later-line treatment in metastatic colorectal cancer patients [abstract no. PD-0022]. Ann Oncol 2007 Jun 1; 18 Suppl. 7:30._Plus poster presented at the 9th World Congress on Gastrointestinal Cancer; 2007 Jun 27–30; Barcelona
Figueras J, Lopez-Ben S, Albiol M, et al. Salvage surgery after neoadjuvant chemotherapy with bevacizumab in unselected patients with advanced CRC and unresectable systemic metastasis: resectability rate and post-operative outcome [abstract no. P-0012]. Ann Oncol 2007 Jun 1; 18 Suppl. 7: 34
Gruenberger B, Scheithauer W, Tamandl D, et al. Effectiveness of neoadjuvant chemotherapy including bevacizumab in patients with resectable colorectal cancer liver metastases [abstract no. 4060]. J Clin Oncol 2007 Jun 20; 25 (18 Suppl.): 178s. Plus poster presented at the 43rd Annual Meeting of the American Society of Clinical Oncology; 2007 Jun 1–5; Chicago (IL)
Reddy SK, Morse MA, Hurwitz HI, et al. Prolonged salvage treatment with bevacizumab for relapse after resection of colorectal liver metastases [abstract no. 182]. 4th Annual Gastrointestinal Cancers Symposium; 2007 Jan 19–21; Orlando (FL)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCormack, P.L., Keam, S.J. Bevacizumab. Drugs 68, 487–506 (2008). https://doi.org/10.2165/00003495-200868040-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200868040-00009