Summary
Abstract
Aprepitant (Emend®) is the first commercially available drug from a new class of agents, the neurokinin NK1 receptor antagonists. Oral aprepitant, in combination with other agents, is indicated for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) associated with highly emetogenic chemotherapy in adults.
In three randomised, double-blind, placebo-controlled trials comparing aprepitant (125mg day 1, 80mg once daily on days 2 and 3 or 2–5) plus standard therapy (intravenous ondansetron and oral dexamethasone) with standard therapy plus placebo, overall complete responses (primary endpoint, defined as no emesis and no rescue therapy) were seen in significantly more patients in the aprepitant arms (63–73% versus 43–52%, p < 0.01 for all comparisons).
Complete responses and complete protection during the acute and delayed phase, and overall complete protection were also observed in significantly more patients in the aprepitant arms. The difference between treatment groups was more marked in the overall and delayed phases than in the acute phase.
The antiemetic efficacy of aprepitant plus standard therapy in the prevention of CINV was maintained for up to six cycles of chemotherapy.
Where assessed, more patients in the aprepitant plus standard therapy arms than the standard therapy plus placebo arms reported no impact of CINV on daily life, as assessed by the Functional Living Index-Emesis.
Aprepitant is generally well tolerated. The most common adverse events in randomised trials were asthenia or fatigue. Other adverse events experienced by aprepitant recipients include anorexia, constipation, diarrhoea, nausea (after day 5 of the study) and hiccups. In addition to being a substrate for cytochrome P450 (CYP) 3A4, aprepitant is also a moderate inhibitor and inducer of this isoenzyme as well as an inducer of CYP2C9. Thus, aprepitant has the potential to interact with other agents metabolised by hepatic CYP isoenzymes. In one trial, there was a higher incidence of serious infection or febrile neutropenia in the aprepitant plus standard therapy arm than the standard therapy plus placebo arm; this was attributed to a pharmacokinetic interaction between aprepitant and dexamethasone. In subsequent trials, a modified dexamethasone regimen was used.
In conclusion, when added to standard therapy (a serotonin 5-HT3 receptor antagonist and a corticosteroid), aprepitant is effective and generally well tolerated in the prevention of CINV associated with highly emetogenic chemotherapy in adults. Despite marked advances in the prevention of CINV, standard therapy does not protect all patients. The addition of aprepitant to standard therapy provides an advance in the prevention of both acute and delayed CINV in adults with cancer.
Pharmacodynamic Properties
Aprepitant is a selective high-affinity neurokinin NK1 receptor antagonist which crosses the blood-brain barrier in humans. Aprepitant competitively binds the NK1 receptors in the brain, thereby blocking the effects of substance P (the natural ligand) in the CNS. The drug has little or no affinity for other neurokinin receptors, rabbit L-type calcium channels, or the receptors targeted by other agents for chemotherapy-induced nausea and vomiting (CINV). In ferrets receiving cisplatin, aprepitant has shown acute- and delayed-phase antiemetic activity and augmented the antiemetic effects of ondansetron and dexamethasone.
Pharmacokinetic Properties
The pharmacokinetics of aprepitant are non-linear across the recommended dose range (recommended regimen 125mg on day 1, 80mg on days 2 and 3). Following this dosage, the area under the concentration-time curve, maximum plasma concentration (Cmax) and time to reach Cmax, respectively, were 19.6 μg · h/mL, 1.6 μg/mL and ≈4 hours on day 1 and 21.2 μg · h/mL, 1.4 μg/mL and ≈4 hours on day 3.
Aprepitant is extensively metabolised in the liver. Based on in vitro data using human liver microsomes, aprepitant is metabolised mainly by cytochrome P450 (CYP) 3A4, and to a lesser extent by CYP1A2 and CYP2C19.
The apparent plasma clearance and apparent terminal elimination half-life ranged from 62 to 90 mL/min and 9 to 13 hours.
Aprepitant does not have clinically relevant effects on the pharmacokinetics of the serotonin 5-HT3 receptor antagonists ondansetron and granisetron. It is unlikely to interact with substrates for the p-glycoprotein transporter.
Aprepitant is a substrate for, and a moderate inhibitor and inducer of, CYP3A4; it is also an inducer of CYP2C9. Coadministration of aprepitant can increase the plasma concentration of CYP3A4 substrates and decrease the plasma concentration of CYP2C9 substrates. Concomitant administration of aprepitant with CYP3A4 inhibitors may increase the plasma concentrations of aprepitant, while concurrent administration with drugs that strongly induce CYP3A4 activity may reduce the plasma aprepitant concentration.
Therapeutic Efficacy
In three multicentre, randomised, double-blind, placebo-controlled trials in adults, aprepitant (125mg day 1, 80mg days 2 and 3 or 2–5) plus standard therapy (intravenous ondansetron and oral dexamethasone) showed efficacy superior to that of standard therapy plus placebo for the primary endpoint, overall complete response (defined as no emesis and no rescue therapy during 0–120 hours postcisplatin, 63–73% versus 43–52%, p < 0.01). Complete responses were also significantly higher with aprepitant plus standard therapy than standard therapy plus placebo during both the acute (83–89% versus 68–78%) and delayed phases (68–75% versus 45–56%).
Complete protection (no emesis, no rescue therapy and no significant nausea) during the overall, acute and delayed phases was observed in significantly more patients in the aprepitant plus standard therapy arms than the standard therapy plus placebo arms (overall 56–65% versus 40–49%, acute 79–85% versus 65–75%, delayed 61–67% versus 41–52%). For both complete response and complete protection, the difference between the treatment arms was numerically greater in the overall and delayed phases than the acute phase.
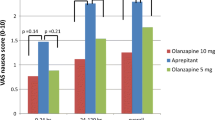
A greater proportion of patients in the aprepitant plus standard therapy arms than in the standard therapy plus placebo arms reported no emetic episodes (overall, acute and delayed, regardless of rescue therapy, p < 0.01). In a phase II study, significantly more patients in the aprepitant arm reported no overall or delayed phase significant nausea (p < 0.01); in the acute phase there was no difference between the treatment groups. In two phase III trials, there was no significant difference between the two treatment arms for overall and delayed phase significant nausea. In these trials, however, significantly more patients in the aprepitant plus standard therapy arms did not require rescue medication (p < 0.05).
The antiemetic efficacy of aprepitant plus standard therapy was maintained for up to six cycles of chemotherapy. In a pooled analysis of two phase III trials, the proportion of patients with no emesis and no significant nausea in the aprepitant plus standard therapy arms was significantly greater than that in the standard therapy plus placebo arms in each treatment cycle (p ≤ 0.006).
In three trials, more aprepitant plus standard therapy recipients than standard therapy plus placebo recipients reported no impact of CINV on daily life, as assessed by the Functional Living Index-Emesis.
Tolerability
In randomised, double-blind trials in adults, aprepitant plus standard therapy was generally well tolerated. Unless stated otherwise, statistical analyses were not reported for tolerability data. The most common adverse event for patients receiving aprepitant plus standard therapy was asthenia/fatigue, which occurred in 17.2–20% of aprepitant plus standard therapy recipients and 9.5–17% of standard therapy plus placebo recipients. Other adverse events occurring in ≥10% of patients in the aprepitant arms were anorexia, constipation, diarrhoea, nausea (after day 5 of the study) and hiccups.
In a dose-finding phase II trial, the incidence of drug-related adverse events in patients receiving aprepitant (125mg day 1, 80mg subsequent days) plus standard therapy was not significantly different to that for those receiving standard therapy plus placebo (27 versus 26%). In two phase III trials, 19.5 and 14.0% of patients in the aprepitant plus standard therapy arms compared with 14.4 and 13.5% in the standard therapy plus placebo arms experienced a drug-related adverse event.
The proportions of patients with a documented serious adverse event were numerically similar in the aprepitant plus standard therapy arms and the standard therapy plus placebo arms of the phase III trials (11.0 versus 9.8% and 16.1 versus 17.0%). In the phase II trial, significantly more patients in the aprepitant plus standard therapy arm than in the standard therapy plus placebo arm experienced serious adverse events (21.5 versus 12.3%, relative risk 1.74, p = 0.032); this difference was attributed to a numerically higher incidence of serious infections or febrile neutropenia in the aprepitant plus standard therapy group. The higher rate of infections in the aprepitant plus standard therapy group was assumed to be because of elevated plasma dexamethasone concentrations, as a result of a pharmacokinetic interaction with aprepitant. In subsequent phase III trials, the dexamethasone regimen was modified and no difference was observed in the incidence of serious infections between treatment arms.
In the three trials, 7.1, 8.0 and 2% of patients in the aprepitant plus standard therapy arms discontinued treatment because of an adverse event, compared with 5.3, 5.3 and 1% in the standard therapy plus placebo arms.
Dosage and Administration
Aprepitant (oral, in combination with a corticosteroid and a serotonin 5-HT3 receptor antagonist) is approved in the US for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy in adults. Aprepitant is not approved for use in children or adolescents. The recommended aprepitant dosage is 125mg administered 1 hour prior to initiating chemotherapy treatment (day 1) and 80mg once daily on days 2 and 3.
Concomitant administration of aprepitant with drugs that are CYP3A4 substrates or inhibitors should be approached with caution because these drugs may interact with aprepitant. Doses of coadministered corticosteroids should be reduced. Coadministration of strong CYP3A4 inducers may decrease the efficacy of aprepitant.
While being treated with aprepitant, patients receiving oral contraceptives should use alternative or back-up contraception and those on long-term warfarin therapy should have their clotting status monitored.
Aprepitant has not been evaluated for longer than 5 days per cycle or for the treatment of established emesis and nausea in patients receiving emetogenic chemotherapy.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Stieler JM, Reichardt P, Riess H, et al. Treatment options for chemotherapy-induced nausea and vomiting: current and future. Am J Cancer 2003; 2(1): 15–26
Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist 2003; 8(2): 187–98
Bender CM, McDaniel RW, Murphy-Ende K, et al. Chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs 2002; 6(2): 94–102
American Society of Health-System Pharmacists. ASHP therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health Syst Pharm 1999 Apr 15; 56: 729–64
Gralla RJ. New agents, new treatments and antiemetic therapy. Semin Oncol 2002 Feb; 29 (1 Suppl. 4): 119–24
European Society of Medical Oncology. Minimum clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting (NV) [online]. Available from URL: http://www.esmo.org/reference/referenceGuidelines/html/MCR13.htm [Accessed 2003 Aug 7]
Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 1999 Sep; 17(9): 2971–94
National Comprehensive Cancer Network. Nausea and vomiting: treatment guidelines for patients with cancer, version 1 [online]. Available from URL: http://www.cancer.org/downloads/CRI/NCCN_nausea.pdf [Accessed 2003 Aug 5]
Hesketh PJ. Potential role of the NK1 receptor antagonists in chemotherapy-induced nausea and vomiting. Support Care Cancer 2001 Jul; 9(5): 350–4
Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 2003 May; 39(8): 1074–80
Sorbera LA, Castaner J, Bayes M, et al. Aprepitant and L-758298: antiemetic, antidepressant, tachykinin NK1 antagonist. Drugs Future 2002; 27(3): 211–22
Diemunsch P, Grelot L. Potential of substance P antagonists as antiemetics. Drugs 2000 Sep; 60(3): 533–46
Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998 Sep 11; 281: 1640–5
Rupniak NMJ, Kramer MS. Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol Sci 1999 Dec; 20(12): 485–90
Rizk AN, Hesketh PJ. Antiemetics for cancer chemotherapy-induced nausea and vomiting: a review of agents in development. Drugs RD 1999 Oct; 2(4): 229–35
Kohler DR, Hughes TE. Pharmacy update: aprepitant (Emend) — review of a new antiemetic agent and guidelines for use at the NIH clinical center [online]. Available from URL: http://www.cc.nih.gov/phar/updates/mayjune03 [Accessed 2003 Sep29]
Merck & Co. Inc. EMEND® (aprepitant) capsules: prescribing information. USA [online]. Available from URL: http://www.merck.com [Accessed 2003 Sep 10]
Tattersall FD, Rycroft W, Cumberbatch M, et al. The novel NK1 receptor antagonist MK-0869 (L-754,030) and its water soluble phosphoryl prodrug, L-758,298, inhibit acute and delayed cisplatin-induced emesis in ferrets. Neuro-pharmacology 2000 Feb 14; 39(4): 652–63
Hale JJ, Mills SG, MacCoss M, et al. Structural optimization affording 2-(R)-(l-(R)-3, 5-bis(trifluoromethyl)-phenylethoxy)-3-(S)-(4-fluoro)phenyl-4-(3-oxo-1,2,4-triazol-5-yl)methylmorpholine, a potent, orally active, long-acting morpholine acetal human NK-1 receptor antagonist. J Med Chem 1998 Nov 5; 41(23): 4607–14
Hale JJ, Mills SG, MacCoss M, et al. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J Med Chem 2000 Mar 23; 43(6): 1234–41
Majumdar AK, McCrea JB, Panebianco DL, et al. Effects of aprepitant on cytochrome P450 3A4 activity using midazolam as a probe. Clin Pharmacol Ther 2003 Aug; 74(2): 150–6
McCrea JB, Majumdar AK, Goldberg MR, et al. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther 2003 Jul; 74(1): 17–24
Blum RA, Majumdar A, McCrea J, et al. Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects. Clin Ther 2003 May; 25: 1407–19
Feuring M, Lee Y, Orlowski LH, et al. Lack of effect of aprepitant on digoxin pharmacokinetics in healthy subjects. J Clin Pharmacol 2003 Aug; 43(8): 912–7
Merck Sharp and Dohme Ltd (UK). Emend: summary of product characteristics [online]. Available from URL: http://www.emea.eu.int/humandocs/Humans/EPAR/emend/emend.htm [Accessed 2003 Nov 25]
Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 2003 May 1; 97(9): 2290–300
de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 2003 Nov 15; 21(22): 4105–11
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer 2003 Jun 15; 97(12): 3090–8
Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist: L-754,030 Antiemetic Trials Group. N Engl J Med 1999 Jan 21; 340(3): 190–5
Van Belle S, Lichinitser MR, Navari RM, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869. Cancer 2002 Jun 1; 94(11): 3032–41
Campos D, Pereira JR, Reinhardt RR, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 2001 Mar 15; 19(6): 1759–67
Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomised, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin — the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003 Nov 15; 21(22): 4112–9
Cocquyt V, Van Belle S, Reinhardt RR, et al. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer 2001 May; 37(7): 835–42
Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control: experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer 2003 Jul; 39(10): 1395–401
Grunberg SM, Hesketh PJ, Carides AD, et al. Relationships between the incidence and control of cisplatin-induced acute vomiting and delayed vomiting: analysis of pooled data from two phase III studies of the NK-1 antagonist aprepitant [abstract no. 2931]. Proc Am Soc Clin Oncol 2003; 22: 729
de Wit R, Herrstedt J, Rapoprt B, et al. The oral NK1 antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of 2 randomised, placebo-controlled clinical trials. Eur J Cancer 2004; 40: 403–10
Martin AR, Ma JG, Carides AD, et al. The oral NK1 antagonist, aprepitant, was effective in maintaining patients’ daily life activities in both male and female patients receiving highly emetogenic chemotherapy [abstract no. 3016]. Proc Am Soc Clin Oncol 2003 May 31; 22: 750
European Society of Medical Oncology (ESMO). Recommendations for prophylaxis of chemotherapy-induced nausea and vomiting (NV). Ann Oncol 2001 Aug; 12(8): 1059–60
Cancer Care Ontario practice guidelines initiative: use of 5-HT3 receptor antagonists in patients receiving moderately or highly emetogenic chemotherapy [online]. Available from URL: http://www.cancercare.on.ca/pdf/fulll2_3.pdf [Accessed 2003 Sep 15]
Markman M. Progress in preventing chemotherapy-induced nausea and vomiting. Cleve Clin J Med 2002 Aug; 69(8): 609–10, 612, 615–7
Jantunen IT, Kataja VV, Muhonen TT. An overview of randomised studies comparing 5-HT3 receptor antagonists to conventional anti-emetics in the prophylaxis of acute chemotherapy-induced vomiting. Eur J Cancer 1997 Jan; 33(1): 66–74
Oettle H, Riess H. Treatment of chemotherapy-induced nausea and vomiting. J Cancer Res Clin Oncol 2001; 127(6): 340–5
MGI Pharma Inc. Aloxi palonosetron HCl injection: prescribing information (USA) [online]. Available from URL: http://www.aloxi.com/downloads/Aloxi_Prescribing_Information.pdf [Accessed 2003 Sep 1]
Marek C. Antiemetic therapy in patients receiving cancer chemotherapy. Oncol Nurs Forum 2003; 30(2): 259–71
Merck. Emend drug interaction studies advised by Cmte, approval supported. FDC Pink 2003 Mar 10; 65: 14
Stahl SM. The ups and downs of novel antiemetic drugs. Part 1: substance P, 5-HT, and the neuropharmacology of vomiting. J Clin Psychiatry 2003 May; 64(5): 498–9
Kris M. Why do we need another antiemetic? Just ask. J Clin Oncol 2003 Nov 15; 21(22): 4077–80
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: P. Diemunsch, Service d’Anesthesie Reanimation Chirurgicale, Les Hopitaux Univeristaires de Strasbourg, Strasbourg, France; S.M. Grunberg, Division of Medical Oncology, University of Vermont, College of Medicine, Burlington, Vermont, USA; M. Markman, Department of Hematology/Oncology, The Cleveland Clinic Taussig Cancer Center, Cleveland, Ohio, USA; W.C. Mertens, Divisions of Hematology, Oncology and Healthcare Quality, Baystate Regional Cancer Program, Springfield, Massachusetts, USA; K. Münstedt, Department of Obstetrics and Gynecology, Universitätklinikum Giessen, Giessen, Germany; R. Navari, Walther Cancer Research Center, University of Notre Dame, Notre Dame, Indiana, USA; F.M. Schnell, Central Georgia Hematology and Oncology Associates, Macon, Georgia, USA; S. Walton, Department of Pharmacy, Northside Hospital Forsyth, Cumming, Georgia, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on aprepitant, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘aprepitant’ or ‘L-754030’ or MK-0869’ or ‘MK-869’. EMBASE search terms were ‘aprepitant’ or ‘L-754030’ or ‘MK-0869’ or ‘MK-869’. AdisBase search terms were ‘aprepitant’ or ‘MK-0869’ or ‘MK-869’. Searches were last updated 15 February 2004.
Selection: Studies in patients treated with highly emetogenic chemotherapy who received aprepitant. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Aprepitant, chemotherapy-induced nausea and vomiting, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Dando, T.M., Perry, C.M. Aprepitant. Drugs 64, 777–794 (2004). https://doi.org/10.2165/00003495-200464070-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464070-00013