Summary
Synopsis
Epirubicin, an anthracycline antitumour antibiotic which is structurally related to doxorubicin, is among the most active single agents used in the management of patients with breast cancer. The drug may be administered alone or in combination with other agents both to patients with early breast cancer and to those with metastatic disease.
There is a clear relationship between epirubicin dose and tumour response. Dose intensified regimens have produced improved response rates in patients with advanced breast cancer compared with standard dose therapy; however, improved overall survival has not yet been demonstrated. The combination of epirubicin with newer agents such as vinorelbine or paclitaxel shows considerable promise, as does the use of epirubicin in high dose regimens with peripheral blood progenitor cell support.
The major adverse effects of epirubicin are acute dose-limiting haematological toxicity and cumulative dose-related cardiac toxicity. These effects are less severe after epirubicin administration than after equimolar doses of doxorubicin. Other major adverse effects of epirubicin administration include mucositis, nausea and vomiting, reversible alopecia and local cutaneous and vesicant reactions.
In summary, epirubicin has an established role in the treatment of both early and advanced breast cancer. In combination with other highly active agents or in dose intensified regimens administered with haemopoietic growth factor and/or peripheral blood progenitor cell support, epirubicin may play a significant role in emerging breast cancer treatment strategies.
Pharmacodynamic Properties
The pharmacodynamic properties of epirubicin are similar to those of other anthracycline antitumour antibiotics. Epirubicin is most active in S and G2 phases of the cell cycle, although the drug exhibits activity in all phases of the cell cycle. After intercalation between DNA base pairs, epirubicin stabilises the topoisomerase II-DNA complex, resulting in irreversible DNA strand breakage. Epirubicin is cytotoxic in vitro and in vivo to breast cancer and other human tumour cells, and cell kill increases with increasing drug concentration.
Tumour resistance to epirubicin and cross-resistance between epirubicin and other antineoplastic agents may occur as a result of several mechanisms, particularly P-glycoprotein—mediated multidrug resistance. A number of drugs, including lonidamine, verapamil, quinidine and tamoxifen attenuate in vitro epirubicin resistance. In clinical studies, lonidamine administered with epirubicin improved response rates, but not survival; the addition of verapamil or quinidine did not improve outcomes.
Epirubicin is toxic to mammalian haemopoietic cells and cardiac tissue. In vitro, epirubicin causes less haematological and cardiac toxicity than equimolar doses of doxorubicin. The iron chelator dexrazoxane reduced epirubicin-induced cardiac damage in rats.
Pharmacokinetic Properties
After bolus intravenous administration, epirubicin undergoes triphasic elimination from the plasma. Its terminal plasma elimination half-life in patients with cancer is 18 to 45 hours. The drug has a large volume of distribution and is concentrated in a variety of normal and cancerous tissues. Epirubicin undergoes extensive hepatic metabolism to epirubicinol and aglycone and glucuronide metabolites. Predictably, plasma clearance is reduced in patients with hepatic dysfunction. Six to 7% of epirubicin is eliminated renally as unchanged drug, and approximately 35% of an administered dose undergoes biliary excretion.
The pharmacokinetics of epirubicin and doxorubicin are similar, although epirubicin is metabolised by different pathways and has a shorter terminal plasma elimination half-life than doxorubicin. Mean area under the plasma concentration-time curve (AUC) values for doxorubicin are higher by a factor of 1.3 to 1.7 than AUC values for equimolar doses of epirubicin.
Therapeutic Efficacy in Breast Cancer
Objective response rates to standard doses of epirubicin monotherapy (≤90 mg/m2 every 3 weeks) in previously treated patients with advanced breast cancer ranged from 16 to 50%. The combination of epirubicin with newer antineoplastics such as paclitaxel or vinorelbine in small, noncomparative studies in patients with advanced breast cancer has produced promising preliminary results (response rates 44 to 83%). A clear relationship exists between epirubicin dose and response rate; however, improved survival as a result of dose intensification [with or without haemopoietic growth factor (HGF) support] has not yet been demonstrated in patients with early breast cancer.
Few comparative studies with epirubicin or other agents in patients with advanced breast cancer have demonstrated significant survival differences between treatment groups; median survival in these patients is generally less than 2 years. Epirubicin 50 to 75 mg/m2 plus cyclophosphamide and fluorouracil (FEC) administered as single doses every 3 weeks is an active and well-studied regimen in patients with advanced breast cancer. FEC is at least as active as the combination of cyclophosphamide, methotrexate and fluorouracil (CMF); it has produced better objective response rates in some studies and superior relapse-free survival in subgroups of patients in other studies. FEC and CMF have similar effects on quality of life.
Patients with metastatic breast cancer who have received prior adjuvant chemotherapy respond less well to chemotherapy for metastatic disease, although the type of regimen (FEC or CMF) administered in the adjuvant setting does not seem to affect response rates. In equimolar or equimyelosuppressive doses, alone or in combination with other agents, epirubicin and doxorubicin produced similar response rates and survival in patients with advanced breast cancer. Patients who fail to respond to first-line chemotherapy for advanced breast cancer may respond to salvage therapy, but response rates are generally below 10%. In noncomparative studies, the combination of epirubicin plus ifosfamide showed promise as a salvage regimen.
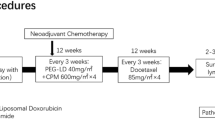
Adjuvant chemotherapy improves survival in patients with early, operable breast cancer. Compared with CMF chemotherapy, the combination of epirubicin plus cyclophosphamide and fluorouracil produced better relapse-free survival and equivalent overall survival in node-positive premenopausal patients. Improved survival as a result of increased dose intensity of epirubicin-containing adjuvant regimens has not yet been demonstrated; however, increased dose intensity positively affected disease-free survival. Epirubicin and tamoxifen produce independent and additive survival benefits in postmenopausal patients with node-positive early breast cancer. Neoadjuvant epirubicin-based regimens produce tumour shrinkage, which permits conservative surgery in a majority of patients; whether this affects long term survival is unknown.
The administration of HGFs permits dose intensification of epirubicin therapy. Because of the steep dose-response curve with epirubicin and other antineoplastic drugs observed in patients with breast cancer, high dose chemotherapy administered with peripheral blood progenitor cell (PBPC) support is of considerable interest. When administered in combination with HGFs, epirubicin is an effective PBPC-mobilising agent. The drug has also been incorporated into both the induction and consolidation phases of high dose regimens. The feasibility of these regimens has been demonstrated, but response and survival data are not yet available.
Tolerability
Bone marrow suppression is the major acute dose-limiting epirubicin toxicity. Neutropenia (<1 × 109 cells/L) occurs most frequently; thrombocytopenia and anaemia occur less often. Neutropenia occurred in 12% of patients who received FEC (epirubicin 50 mg/m2 on days 1 and 8) every 3 weeks. Combinations containing high doses of epirubicin (≥200 mg/m2/dose) cause neutropenia in all patients, but PBPC infusions facilitate complete haematological recovery. On an equimolar basis, epirubicin produces less haematological toxicity than doxorubicin.
Epirubicin in high cumulative doses can cause chronic irreversible cardiomyopathy leading to congestive heart failure that is qualitatively identical to that sometimes seen after high cumulative doses of doxorubicin. The maximum recommended cumulative epirubicin dose is 1000 mg/m2, considerably higher than the recommended maximum cumulative doxorubicin dose (450 to 550 mg/m2). Cardiac monitoring is recommended prior to each treatment cycle in patients with concomitant cardiac risk factors. Administration of an ACE inhibitor was shown to reduce the severity of epirubicin-induced congestive heart failure. The cardio-protectant dexrazoxane has also demonstrated promising results in clinical trials.
Other important adverse effects of epirubicin include mucositis, nausea and vomiting, reversible alopecia and local cutaneous reactions. Mucositis generally parallels haematological toxicity and can be dose limiting with high dose epirubicin administration. Without prophylactic antiemetics, nausea and vomiting occur in >50% of patients who receive epirubicin 50 to 75 mg/m2 single doses, but the incidence is lower in patients pretreated with antiemetic agents. Secondary leukaemias have occurred in epirubicin-treated breast cancer patients, particularly those who received concomitant alkylating agent therapy; however, the actual risk of leukaemogenesis is not known.
Dosage and Administration
Epirubicin is administered intravenously. Epirubicin monotherapy is commonly administered in single doses of 75 to 90 mg/m2 every 3 weeks; with other anti-neoplastic drugs, doses of 50 to 60 mg/m2 every 3 weeks are administered. Increasingly, higher doses are used. As monotherapy, epirubicin doses as high as 180 mg/m2 have been administered at 3-week intervals in noncomparative studies, but more commonly, the maximum tolerated dose without HGF support is 120 mg/m2. In combination with cyclophosphamide and fluorouracil, epirubicin 60 mg/m2 on days 1 and 8 every 4 weeks is an effective adjuvant chemotherapy regimen. With the support of growth factor and PBPC administration, epirubicin 200 mg/m2 has been administered in combination with other antineoplastic agents at weekly intervals.
Dosage reduction is suggested in patients with moderate or severe hepatic dysfunction. Because of the risk of cardiotoxicity at higher doses, the usual recommended maximum lifetime cumulative dose of epirubicin is 1000 mg/m2.
Similar content being viewed by others
References
Mross K, Maessen P, van der Vijgh WJF, et al. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol 1988; 6: 517–26
Plosker GL, Faulds D. Epirubicin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 1993 May; 45: 788–856
Berens ME, Saito T, Welander CE, et al. Antitumor activity of new anthracycline analogues in combination with interferon alfa. Cancer Chemother Pharmacol 1987; 19: 301–6
Capranico G, Zunino F, Kohn K, et al. Sequence-selective topoisomerase II inhibition by anthracycline derivatives in SV40 DNA: relationship with DNA binding affinity and cytotoxicity. Biochemistry 1990; 29: 562–9
Pauwels O, Pasteels J-L, Atassi G, et al. In vitro digital cell image analysis of morphonuclear modifications induced by natural DNA-interacting anticancer drugs in three neoplastic cell lines. Methods Find Exp Clin Pharmacol 1995 Apr; 17: 151–62
Larsson R, Nygren P. Cytotoxic activity of topoisomerase II inhibitors in primary cultures of tumor cells from patients with human hematologic and solid tumors. Cancer 1994 Nov 15; 74: 2857–62
Kaye SB. Reversal of multidrug resistance. Cancer Treat Rev 1990; 17 Suppl. A: 37–43
Ravdin PM. Anthracycline resistance in breast cancer: clinical applications of current knowledge. Eur J Cancer 1996; 31A Suppl. 7: S11–4
Muss HB, Thor AD, Berry DA, et al. c-erbB-2 Expression and responsive to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 1994; 330: 1260–6
Revillion F, Hebbar M, Bonneterre J, et al. Plasma c-erbB2 concentrations in relation to chemotherapy in breast cancer patients. Eur J Cancer 1996; 32A(2): 231–4
Hargrave RM, Davey MW, Davey RA, et al. Development of drug resistance is reduced with idarubicin relative to other anthracyclines. Anticancer Drugs 1995 Jun; 6: 432–7
Dogliotti L, Berruti A, Buniva T, et al. Lonidamine significantly increases the activity of epirubicin in patients with advanced breast cancer: results from a multicenter prospective randomized trial. J Clin Oncol 1996; 14: 1165–72
Pacini P, Algeri R, Rinaldini M, et al. FEC (fluorouracil, epirubicin and cyclophosphamide) versus EM (epirubicin and mitomycin-C) with or without lonidamine as first line treatment for advanced breast cancer. A multicentric randomized study. Preliminary report. Int J Oncol 1994 Mar; 4: 761–6
Mross K, Bohn C, Edler L, et al. Randomized phase II study of single-agent epirubicin +/− verapamil in patients with advanced metastatic breast cancer. An AIO clinical trial. Arbeitsgemeinschaft Internistische Onkologie of the German Cancer Society. Ann Oncol 1993 Jan; 4: 45–50
Wishart GC, Bissett D, Paul J, et al. Quinidine as a resistance modulator of epirubicin in advanced breast cancer: mature results of a placebo-controlled randomized trial. J Clin Oncol 1994 Sep; 12: 1771–7
Rzymowska J, Dyrda Z. Enzyme activities and level of SH groups in breast carcinomas. Int J Biochem 1993 Sep; 25: 1331–4
Millward MJ, Harris AL, Cantwell BMJ. Phase II study of doxorubicin plus ifofamide/mesna in patients with advanced breast cancer. Cancer 1990; 65: 2421–5
Tidefelt U, Sundman-Engberg B, Paul C. Intracellular uptake and cytotoxic effect in vitro of doxorubicin and epirubicin in human leukemic and normal hematopoietic cells. Cancer Chemother Pharmacol 1991; 29: 7–12
Bonadonna G, Gianni L, Santoro A, et al. Drugs ten years later: epirubicin. Ann Oncol 1993 May; 4: 359–69
Pouna P, Bonoron-Adèle S, Gouverneur G, et al. Evaluation of anthracycline cardiotoxicity with the model of isolated, perfused rat heart: comparison of new analogues versus doxorubicin. Cancer Chemother Pharmacol 1995 Jan; 35: 257–61
Pouna P, Bonoron-Adèle S, Gouverneur G, et al. Development of the model of rat isolated perfused heart for the evluation of anthracycline cardiotoxicity and its circumvention. Br J Pharmacol 1996; 117: 1593–9
de-Jong J, Schoofs PR, Snabilié AM, et al. The role of biotransformation in anthracycline-induced cardiotoxicity in mice. J Pharmacol Exp Ther 1993 Sep; 266: 1312–20
Praet M, Ruysschaert J-M. In-vivo and in-vitro mitochondrial membrane damages induced in mice by adriamycin and derivatives. Biochim Biophys Acta 1993 Jun 18; 1149: 79–85
Lilenbaum RC, Green MR. Novel chemotherapeutic agents in the treatment of non-small-cell lung cancer. J Clin Oncol 1993 Jul; 11: 1391–402
Robert J. Clinical pharmacokinetics of epirubicin. Clin Pharmacokinet 1994 Jun; 26: 428–38
Robert J, Bui NB. Pharmacokinetics and metabolism of epirubicin administered as i.v. bolus and 48-h infusion in patients with advanced soft-tissue sarcoma. Ann Oncol 1992 Sep; 3: 651–6
Dobbs NA, Twelves CJ. Epirubicin (epi) dosage: ending surface area normalisation would not increase variability in pharmacokinetics (PK) or pharmacodynamics (PD) [abstract no. 1501]. Proc Am Soc Clin Oncol 1996; 15: 474
Schott B, Robert J. Comparative activity of anthracycline 13-dihydrometabolites against rat glioblastoma cells in culture. Biochem Pharmacol 1988; 22: 4069–74
Camaggi CM, Strocchi E, Tamassia V, et al. Pharmacokinetic studies of 4′-epidoxorubicin in cancer patients with normal and impaired renal function and with hepatic metastases. Cancer Treat Rep 1982; 66: 1819–24
Cosolo WC, Morgan DJ, Seeman E, et al. Lean body mass, body surface area and epirubicin kinetics. Anticancer Drugs 1994 Jun; 5: 293–7
Bastholt L, Dalmark M, Gjedde S, et al. Dose-relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: A randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol 1996; 14(4): 1146–55
Tjuljandin SA, Doig RG, Sobol MM, et al. Pharmacokinetics and toxicity of two schedules of high dose epirubicin. Cancer Res 1990; 50: 5095–101
Mross K, Hamm K, Hossfeld DK. Effects of verapamil on the pharmacokinetics and metabolism of epirubicin. Cancer Chemother Pharmacol 1993; 31: 369–75
Jakobsen P, Sørensen B, Bastholt L, et al. The pharmacokinetics of high-dose epirubicin and of the cardioprotector ADR-529 given together with cyclophosphamide, 5-fluorouracil, and tamoxifen in metastatic breast-cancer patients. Cancer Chemother Pharmacol 1994 Nov; 35: 45–52
Czejka M, Bandak S, Simon D, et al. Pharmacokinetic interaction between 4′-epidoxorubicin and the multidrug resistance reverting agent quinine. Z Naturforsh C 1995 Jul–Aug; 50: 565–70
McGuire TR. Breast Cancer. In: DiPiro JT, Talbert RL, editors. Pharmacotherapy: a pathophysiologic approach. 2nd ed. Norwalk, Connecticut: Appleton & Lange, 1993: 1930–45
Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31000 recurrences and 24000 deaths among 75000 women (Pt 1). Lancet 1992; 339(8784): 1–15
Hortobagyi GN. Comprehensive management of locally advanced breast cancer. Cancer 1990; 66 Suppl.: 1387–91
Hayward JL, Rubens RD, Carbone PP, et al. Assessment of response to therapy in advanced breast cancer: a project of the programme on clinical oncology of the International Union Against Cancer, Geneva, Switzerland. Br J Cancer 1977; 35: 292–8
Seidman AD. Chemotherapy for advanced breast cancer: A current perspective. Semin Oncol 1996 February; 23(1) Suppl. 2: 55–9
Cook AM, Chambers EJ, Rees GJG. Comparison of mitozantrone and epirubicin in advanced breast cancer [abstract]. Br J Cancer 1995 Jul; 72 Suppl. 25: 34
Hausmaninger H, Lehnert M, Steger G, et al. Randomised phase II study of epirubicin-vindesine versus mitoxantronevindesine in metastatic breast cancer. Eur J Cancer A 1995; 13A(13–14): 2169–73
Heidemann E, Steinke B, Hartlapp J, et al. Prognostic sub-groups: the key factor for treatment outcome in metastatic breast cancer: results of a three-arm randomized multicenter trial comparing doxorubicin, epirubicin and mitoxantrone each in combination with cyclophosphamide. Onkologie 1993 Oct; 16: 344–53
Lawton PA, Spittle MF, Ostrowski MJ, et al. A comparison of doxorubicin, epirubicin and mitozantrone as single agents in advanced breast carcinoma. Clin Oncol R Coll Radiol 1993; 5: 80–4
Pavesi L, Preti P, Da Prada G, et al. Epirubicin versus mitoxantrone in combination chemotherapy for metastatic breast cancer. Anticancer Res 1995 Mar–Apr; 15: 495–501
Colajori E, Ackland S, Anton A, et al. I.V. FEC with epirubicin (E) 50 mg/m2 D1,8 prolongs time to progression (TTP) with respect of I.V. CMF D1,8 given at equimyelosuppressive doses as front line chemotherapy of metastatic breast cancer: a randomized multinational multicentric phase III trial [abstract]. Proc Am Soc Clin Oncol 1995 Mar; 14: 114
Fraser SCA, Dobbs HJ, Ebbs SR, et al. Combination or mild single agent chemotherapy for advanced breast cancer? CMF vs epirubicin measuring Quality of Life. Br J Cancer 1993 Feb; 67: 402–6
Catimel G, Spielmann M, Dieras V, et al. Phase I study of paclitaxel and epirubicin in patients with metastatic breast cancer: A preliminary report. Semin Oncol 1996; 23(1) Suppl. 1: 24–7
Conte P, Michelotti A, Baldini E, et al. Activity and safety of epirubicin plus paclitaxel in advanced breast cancer. Semin Oncol 1996 Feb; 23(1) Suppl. 1: 28–32
Lück H-J, Thomssen C, du Bois A, et al. Phase II study of paclitaxel and 4-epi-doxorubicin (epirubicin) as first-line therapy in patients with metastatic breast cancer [abstract no. 147]. Proc Am Soc Clin Oncol 1996; 15: 120
Chadjaa M, Izzo J, May-Levin F, et al. Preliminary data on 4′epiadriamycine (EPI)-vinorelbine (VNB): A new active combination in advanced breast cancer [abstract no. 152]. Proc Am Soc Clin Oncol 1993; 12: 88
Meriggi F, Zaniboni A, Arcangeli G, et al. VELF: an active outpatient regimen for aggressive metastatic breast cancer (MBC). Preliminary results [abstract no. 106]. Eur J Cancer 1994; 30A Suppl. 1: S22
Nisticò C, Garufi C, Ranuzzi M, et al. High activity in advanced breast cancer (ABC) with the weekly combination of epirubicin (EPI), vinorelbine (VNR) and G-CSF [abstract no. 228]. Proc Am Soc Clin Oncol 1996; 15: 141
Gurney H, Harnett P, Stuart-Harris R, et al. Continuous infusion of vincristine, ifosfamide and epirubicin over 6 weeks in treatment-resistant advanced breast cancer. Eur J Cancer A 1995 Oct; 31A: 1773–7
Loeffler TM, Freund W, Hausamen TU. Ambulatory continuous infusion ifosfamide/mesna and epirubicin in pretreated metastatic breast cancer. Evidence for improved response and median survival [abstract]. Breast Cancer Res Treat 1996; 37 Suppl.: 44
Mosconi AM, Gori S, Colozza M, et al. Phase II study of epirubicin and ifosfamide/mesna in patients with advanced breast cancer [abstract]. Proc Am Soc Clin Oncol 1994 Mar; 13: 109
Blomqvist C, Elomaa I, Rissanen P, et al. Influence of treatment schedule on toxicity and efficacy of cyclophosphamide, epirubicin, and fluorouracil in metastatic breast cancer: a randomized trial comparing weekly and every-4-week administration. J Clin Oncol 1993 Mar; 11: 467–73
Colajori E, Tosello C, Pannuti F, et al. Randomized multinational trial comparing epirubicin (Epi) 50 mg/m2 vs 100 mg/m2 in combination with 5-fluorouracil (5FU) and cyclophosphamide (CTX) as front line treatment of metastatic breast cancer (MBC) [abstract no. 0127]. Ann Oncol 1994; 5 Suppl. 8: 3023
Focan C, Andrien JM, Closon MT, et al. Dose-response relationship of epirubicin-based first-line chemotherapy for advanced breast cancer: a prospective randomized trial. J Clin Oncol 1993 Jul; 11: 1253–63
Marschner N, Kreienberg R, Souchon R, et al. Evaluation of the importance and relevance of dose intensity using epirubicin and cyclophosphamide in metastatic breast cancer: interim analysis of a prospective randomized trial. Semin Oncol 1994 Feb; 21 Suppl. 1: 10–6
Neri B, Pacini P, Algeri R, et al. Conventional versus high-dose epidoxorubicin as single agent in advanced breast cancer. Cancer Invest 1993; 11(2): 106–12
Riccardi A, Giordano M, Brugnatelli S, et al. Different doses of epirubicin associated with fixed doses of cyclophosphamide and 5-fluorouracil: a randomised study in advanced breast cancer. Eur J Cancer A 1995; 31A(9): 1549–51
Venturini M, Bruzzi P, Del Maestro L, et al. Effect of adjuvant chemotherapy with or without anthracyclines on the activity and efficacy of first-line cyclophosphamide, epidoxorubicin, and fluorouracil in patients with metastatic breast cancer. J Clin Oncol 1996 Mar; 14(3): 764–73
Fraser SCA, Ramirez AJ, Ebbs SR, et al. A daily diary for quality of life measurement in advanced breast cancer trials. Br J Cancer 1993 Feb; 67: 341–6
Ejlertsen B, Pfeiffer P, Pedersen D, et al. Decreased efficacy of cyclophosphamide, epirubicin and 5-fluorouracil in metastatic breast cancer when reducing treatment duration from 18 to 6 months. Eur J Cancer 1993; 29A: 527–31
Jones AL, Smith IE, O’Brien MER, et al. Phase II study of continuous infusion fluorouracil with epirubicin and cisplatin in patients with metastatic and locally advanced breast cancer: an active new regimen. J Clin Oncol 1994 Jun; 12: 1259–65
Bonnefoi H, Smith IE, O’Brien MER, et al. Phase II study of continuous infusional 5-fluorouracil with epirubicin and carboplatin (instead of cisplatin) in patients with metastatic/locally advanced breast cancer (infusional ECarboF): a very active and well-tolerated outpatient regimen. Br J Cancer 1996 Feb; 73: 391–6
Porkka K, Blomqvist C, Rissanen P, et al. Salvage therapies in women who fail to respond to first-line treatment with fluorouracil, epirubicin, and cyclophosphamide for advanced breast cancer. J Clin Oncol 1994 Aug; 12: 1639–47
Gardin G, Campora E, Gasco M, et al. Unclear value of salvage chemotherapy after failure to first-line 5-fluorouracil, epirubicin, cyclophosphamide (FEC) regimen for metastatic breast cancer [abstract no. 225]. Proc Am Soc Clin Oncol 1996; 15: 140
Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol 1984; 2(11): 1281–8
Romito S, Giotta F, Maggi V, et al. 120 Hour continuous infusion of 4-epidoxorubicin (4-EPI) in untreated patients with advanced breast cancer. A pilot study: preliminary data [abstract no. 336]. Ann Oncol 1992 Nov; 3 Suppl. 5: 87
Robert J. Epirubicin: clinical pharmacology and dose-effect relationship. Drugs 1993; 45 Suppl. 2: 20–30
van der Wall E, Rutgers EJT, Holtkamp MJ, et al. Efficacy of up-front 5-fluorouracil-epidoxorubicin-cyclophosphamide (FEC) chemotherapy with an increased dose of epidoxorubicin in high-risk breast cancer patients. Br J Cancer 1996; 73: 1080–5
Vici P, Botti F, Conti L, et al. CEF ± G-CSF as primary chemotherapy (PCT) in ≥ 3cm breast carcinoma (BC) [abstract no. 111]. Proc Am Soc Clin Oncol 1996; 15: 111
Lalisang R, Wils J, Nortier J, et al. A comparative study of dose escalation versus interval reduction to obtain dose intensification of epirubicin and cyclosphosphamide with G-CSF (filgastrim) for patients with metastatic breast cancer [abstract]. Proc Am Soc Clin Oncol 1994 Mar; 13: 60
Cognetti F, Scinto AF, Cercato MC, et al. Phase II trial of high dose epirubicin and cyclophosphamide every two weeks + r-met-HuG-CSF in locally advanced and metastatic breast cancer [abstract]. Proc Am Soc Clin Oncol 1994 Mar; 13: 88
Neijens V, ten Hoeve R, Valdes OR, et al. Feasibility study of the combination of high dose epirubicin and cyclophosph-amide and G-CSF every two weeks together with ICRF 187 [abstract no. 429]. Ann Oncol 1994; 5 Suppl. 5: 182
Piccart MJ, Bruning P, Wildiers J, et al. An EORTC pilot study of filgrastim (recombinant human granulocyte colony stimulating factor) as support to a high dose-intensive epiadriamycin-cyclophosphamide regimen in chemotherapy-naive patients with locally advanced or metastatic breast cancer. Ann Oncol 1995 Sep; 6: 673–7
Scinto AF, Cercato MC, Botti C, et al. Phase II trial of high-dose epirubicin and cyclophosphamide in advanced breast cancer. Eur J Cancer A 1994; 30A(9): 1285–8
Sledge GWJ. Adjuvant therapy for early stage breast cancer. Semin Oncol 1996 Feb; 23(1) Suppl. 2: 51–4
Goldhirsch A, Wood WC, Senn H-J, et al. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst 1995 Oct 4; 87: 1441–5
Levine M, Bramwell V, Bowman D, et al. A clinical trial of intensive CEF versus CMF in premenopausal women with node positive breast cancer [abstract no. 112]. Proc Am Soc Clin Oncol 1995 Mar; 14: 103
Coombes RC, Bliss JM, Wils J, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. J Clin Oncol 1996 Jan; 14: 35–45
De Placido S, Perrone F, Carlomagno C, et al. CMF vs. alternating CMF/EV in the adjuvant treatment of operable breast cancer. A single centre randomised clinical trial (Naples Gun-3 study). Br J Cancer 1995; 71: 1283–7
Bonneterre J, Roché H, Bremond A, et al. A randomized trial of adjuvant chemotherapy with FEC 50 vs FEC 100 for node-positive operable breast cancer early report [abstract no. 82]. Proc Am Soc Clin Oncol 1996 Mar; 15: 104
Brémond A, Kerbrat P, Fumoleau P, et al. Five year follow-up results of a randomized trial testing the dose intensity and duration of chemotherapy in node postive premenopausal breast cancer patients [abstract no. 119]. Proc Am Soc Clin Oncol 1996 Mar; 15: 113
Sertoli MR, Bruzzi P, Pronzato P, et al. Randomized cooperative study of perioperative chemotherapy in breast cancer. J Clin Oncol 1995 Nov; 13: 2712–21
Richards MA, Ramirez AJ, Smith P, et al. Influence of adjuvant chemotherapy on quality of life of patients with breast cancer [abstract]. Anticancer Drugs 1995 Mar; 6 Suppl. 2: 84
Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31000 recurrences and 24000 deaths among 75000 women (Pt 2). Lancet 1992; 339(8785): 71–85
Wils J, Bliss JM, Coombes RC, et al. A multicentre randomized trial of tamoxifen vs. tamoxifen plus epirubicin in postmenopausal women with node-positive breast cancer [abstract no. 101]. Proc Am Soc Clin Oncol 1996 March; 15: 109
Namer M, Fumuleau P, Devaux E, et al. Node positive postmenopausal breast cancer patients: six year follow-up results of a randomized trial testing the role of hormontherapy (HT) chemotherapy (CT) or combination [abstract no. 79]. Proc Am Soc Clin Oncol 1996 March; 15: 103
Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med 1994; 330(18): 1253–9
Aglietta M, Monzeglio C, Pasquino P, et al. Short-term administration of granulocyte-macrophage colony stimulating factor decreases hematopoietic toxicity of cytostatic drugs. Cancer 1993 Nov 15; 72: 2970–3
Findlay B, Tonkin K, Crump M, et al. A dose escalation trial of adjuvant CEF chemotherapy with G-CSF for premenopausal women with node-positive breast cancer [abstract no. 60]. Proc Am Soc Clin Oncol 1996 Mar; 15: 99
Del Mastro L, Garrone O, Sertoli MR, et al. A pilot study of accelerated cyclophosphamide, epirubicin and 5-fluorouracil plus granulocyte colony stimulating factor as adjuvant therapy in early breast cancer. Eur J Cancer 1994; 30A: 606–10
Murias A, Almenarez J, Molano F, et al. Phase II adjuvant high dose FEC (HDFEC) plus G-CSF in breast cancer: preliminary results [abstract no. P56]. Anticancer Drugs 1995 Mar; 6 Suppl. 2: 73
Rosso R, Mig-1 Intergroup. FEC21 vs FEC14 as adjuvant treatment for early breast cancer patients: a phase III multicentric, randomized study [abstract]. Anticancer Drugs 1995 Mar; 6 Suppl. 2: 74
Galligioni E, Cetto G, Nascimben O, et al. Adjuvant chemotherapy with high-dose epirubicin and cyclophosphamide vs CMF in high risk premenopausal breast cancer patients. Prospective randomized phase III trial [abstract]. Breast Cancer Res Treat 1996; 37 Suppl.: 78
Harris L, Swain SM. The role of primary chemotherapy in early breast cancer. Semin Oncol 1996 Feb; 23(1) Suppl. 2: 31–42
van de Velde CJH. Preoperative chemotherapy in operable breast cancer: the influence of timing FEC in relation to surgery. Drugs 1993; 45 Suppl. 2: 31–7
Bonadonna G, Veronesi U, Brambilla C, et al. Primary chemotherapy for resectable breast cancer. Recent Results Cancer Res 1993; 127: 113–7
Llombart-Cussac A, Spielmann M, Antione E, et al. Primary chemotherapy with high-dose FEC regimen and G-CSF to allow conservative treatment for large early breast cancer [abstract]. Breast Cancer Res Treat 1996; 37 Suppl.: 93
Smith IE, Walsh G, Jones A, et al. High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol 1995 Feb; 13: 424–9
Bergh J. High-dose therapy with autologous bone marrow stem cell support in primary and metastatic human breast cancer: a review. Acta Oncol 1995; 34(5): 669–74
de Vries EGE, Rodenhuis S, Schouten HC, et al. Phase II study of intensive chemotherapy with autologous bone marrow transplantation in patients in complete remission of disseminated breast cancer [abstract no. 151]. Proc Am Soc Clin Oncol 1994; 13: 87
Klumpp TR, Goldberg SL, Mangan KF, et al. High dose cyclophosphamide, etoposide, and carboplatin (CEC) with autologous stem cell rescue for chemosensitive metastatic and high risk breast cancer [abstract no. 245]. Proc Am Soc Clin Oncol 1994; 13: 111
von Schilling C, Herrmann F. Dose-intensified treatment of breast cancer: current results. J Mol Med 1995 Dec; 73: 611–27
Rosti G, Albertazzi L, Ferrante P, et al. Epirubicin + G-CSF as peripheral blood progenitor cells (PBPC) mobilising agents in breast cancer patients. Ann Oncol 1995 Dec; 6: 1045–7
Milliken S, Kwan Y, Dalley D, et al. The use of conventional dose epirubicin + GMCSF to obtain peripheral blood stem cells in the treatment of breast cancer with high dose chemotherapy and stem cell rescue [abstract]. Aust N Z J Med 1995 Feb; 25: 70
Marangolo M, Rosti G, Albertazzi L, et al. Epirubicin 150 mg/sqm plus G-CSF as peripheral blood stem cells (PBSC) mobilizing agents in breast cancer patients. A feasibility study [abstract]. Proc Am Soc Clin Oncol 1994 Mar; 13: 107
van der Wall E, Nooijen WJ, Baars JW, et al. High-dose carboplatin, thiotepa and cyclophosphamide (CTC) with peripheral bloodstem cell support in the adjuvant therapy of high-risk breast cancer: a practical approach. Br J Cancer 1995 Apr; 71: 857–62
Venturini M, Del Mastro L, Melioli G, et al. Release of peripheral blood progenitor cells during standard dose cyclophosphamide, epidoxorubicin, 5-fluorouracil regimen plus granulocyte colony stimulating factor for breast cancer therapy. Cancer 1994 Oct 15; 74: 2300–6
Bengala C, Giannessi PG, Baldini E, et al. Epirubicin (EpiADM), paclitaxel (TXL) and G/GM-CSF can mobilize peripheral blood progenitor cells (PBPCs) to support a myelosuppressive treatment in metastatic breast cancer (MBC) [abstract no. 1017]. Proc Am Soc Clin Oncol 1996 Mar; 15: 348
Basser RL, To LB, Begley CG, et al. Adjuvant treatment of high-risk breast cancer using multicycle high-dose chemotherapy and filgrastim-mobilized peripheral progenitor cells. Clin Cancer Res 1995 Jul; 1: 715–21
Danova M, Rosti V, Mora O, et al. The use of peripheral blood hemopoietic progenitors mobilized with standard dose chemotherapy plus granulocyte colony-stimulating factor to support multicyclic dose-intensive chemotherapy for advanced breast cancer. Oncol Rep 1995 Nov–Dec; 2: 1075–8
ten Vergert EM, Rodenhuis S, Bontenbal M, et al. Quality of life in a randomized adjuvant breast carcinoma study with standard versus high dose chemotherapy [abstract no. 77]. Proc Am Soc Clin Oncol 1996 March; 15: 103
Scinto AF, Ferraresi V, Campioni N, et al. Accelerated chemotherapy with high-dose epirubicin and cyclophosphamide plus r-met-HUG-CSF in locally advanced and metastatic breast cancer. Ann Oncol 1995 Sep; 6: 665–71
Hansen F, Stenbygaard L, Skovsgaard T. Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on hematologic toxicity induced by high-dose chemotherapy in patients with metastatic breast cancer. Acta Oncol 1995; 34(7): 919–24
Chevallier B, Chollet P, Merrouche Y, et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. J Clin Oncol 1995 Jul; 13: 1564–71
Pedersen-Bjergaard J, Sigsgaard TC, Nielsen D, et al. Acute monocytic or myelomonocytic leukemia with balanced chromosome translocations to band 11q23 after therapy with 4-epi-doxorubicin and cisplatin or cyclophosphamide for breast cancer. J Clin Oncol 1992; 10(9): 1444–51
Shepherd L, Ottaway J, Myles J, et al. Therapy-related leukemia associated with high-dose 4-epi-doxorubicin and cyclophosphamide used as adjuvant chemotherapy for breast cancer. J Clin Oncol 1994 Nov; 12: 2514–5
Marty M, International CCGSC. Epirubicin and the risk of leukemia: not substantiated? [letter]. J Clin Oncol 1993 Jul; 11: 1431–2
Ragaz J, Yun J, Spinelli J. Analysis of incidence of secondary acute myleogenous leukemias (2ndAML) in breast cancer patients (BCP) treated with adjuvant therapy (AT)-association with therapeutic regimens [abstract no. 147]. Proc Am Soc Clin Oncol 1995; 14: 112
Smith M, Abrams J, Rubinstein L. Surveillance of populations at risk for secondary leukaemias. In: ASCO educational book; 1995 May 20–23. Los Angeles, CA: American Society of Clinical Oncology, 1995: 214–20
Macchiarini P, Chella A, Riva A, et al. Phase II feasibility study of high dose epirubicin-based regimens for untreated patients with small-cell lung cancer. Am J Clin Oncol Cancer Clin Trials 1990; 13(6): 495–500
Jensen BV, Nielsen SL, Skovsgaard T. Treatment with angiotensin-converting-enzyme inhibitor for epirubicin-induced dilated cardiomyopathy. Lancet 1996; 347: 297–9
Weisberg SR, Rosenfeld CS, York RM, et al. Dexrazoxane, (ADR-259, ICRF-187, Zinecard®) protects against doxorubicin induced chronic cardiotoxicity [abstract no. 190]. Proc Am Soc Clin Oncol 1992; 11: 91
Michelotti A, Venturini M, Conte PF, et al. Cardioxane (ICRF-187) protects against epirubicin induced cardiomyopathy in advanced breast cancer (ABC) patients: a phase III study [abstract no. 91]. Proc Am Soc Clin Oncol 1995; 14: 98
Sledge GW Jr, Robert N, Sparano JA, et al. Pacltiaxel (Taxol)/doxorubicin combinations in advanced breast cancer: the Eastern Cooperative Oncology Group experience. Semin Oncol 1994; 21(5) Suppl. 8: 15–8
Holmes FA, Frye D, Valero V, et al. Phase I study of taxol (T) and doxorubicin with G-CSF in patients (Pt) without prior chemotherapy for metastatic breast cancer [abstract no. 66]. Proc Am Soc Clin Oncol 1992; 11: 60
Lück H-J, Thomssen C, duBois A, et al. Interim analysis of a phase II study of epirubicin and paclitaxel as first-line therapy in patients with metastatic breast cancer. Semin Oncol 1996 Feb; 23(1) Suppl. 1: 33–6
Bronchud MH, Howell A, Crowther D, et al. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer 1989; 60: 121–5
Green M. Dose-intensive chemotherapy with cytokine support. Semin Oncol 1994; 1 Suppl. 1: 1–6
Bertelli G, Gozza A, Forno GB, et al. Topical dimethylsulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol 1995; 13: 2851–5
Gianni L, Bonadonna G. Comments on epirubicin [reply]. Ann Oncol 1994; 5: 99
de Valeriola D. Dose optimization of anthracyclines. Anticancer Res 1994 6A, Nov–Dec No.; 14: 2307–14
Twelves CJ, Dobbs NA, Cruickshank C, et al. Adaptive dosing for epirubicin: prospective pharmacokinetic evaluation of nomogram based on serum aspartate aminotransferase [abstract]. Br J Cancer 1995 Apr; 71 Suppl. 24: 45
Twelves CJ, Dobbs NA, Michael Y, et al. Clinical pharmacokinetics of epirubicin: the importance of liver biochemistry tests. Br J Cancer 1992 Oct; 66: 765–9
Farmitalia Carlo Erba. Farmorubicina prescribing information. Milan, Italy, 1989.
Bearman SI, Shpall EJ, Jones RB, et al. High-dose chemotherapy with autologous hematopoietic progenitor cell support for metastatic and high-risk primary breast cancer. Semin Oncol 1996 Feb; 23(1) Suppl. 2: 60–7
Rubens RD, Bajetta E, Bonneterre J, et al. Treatment of relapse of breast cancer after adjuvant systemic therapy — review and guidelines for future research. Eur J Cancer 1994; 30A(1): 106–11
Goldhirsch A, Gelber RD, Castiglione M, et al. Present and future projects of the International Breast Cancer Study Group. Cancer 1994 Aug 1; 74 Suppl.: 1139–49
Bezwoda WR, Seymour L, Dansey RD. High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial. J Clin Oncol 1995; 13: 2483–9
Peters WP, Ross M, Vredenburgh JJ, et al. High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high-risk primary breast cancer. J Clin Oncol 1993; 11(6): 1132–43
McCarthy M. Unproven breast-cancer therapy widely used in USA. Lancet 1996; 347: 1617
de Vries EGE, ten Vergert EM, Mastenbroek CG, et al. Breast cancer studies in the Netherlands [letter]. Lancet 1996; 348: 407–8
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: R.L. Basser, Department of Clinical Haematology and Medical Oncology, Royal Melbourne Hospital, Parkville, Victoria, Australia; P.F. Conte, U.O. Oncologia Medica, Ospedale S. Chiara, Pisa, Italy; C. Focan, Centre Hospitalier Saint-Joseph-Espérance, Liège, Belgium; K. Hatake, Division of Hematology, Department of Medicine, Jichi Medical School, Minamikawachi, Kawachi, Tochigi, Japan; R. Larsson, Department of Clinical Pharmacology, Uppsala University Hospital, Uppsala, Sweden; M. Levine, Ontario Cancer Treatment and Research Foundation, Hamilton Regional Cancer Centre, Hamilton, Ontario, Canada; D. Porter, Auckland Hospital, Auckland, New Zealand; J. Robert, Département de Biochemie Médicale et Biologie Moléculaire, Université de Bordeaux, Bordeaux, France; A. Tomiak, London Regional Cancer Centre, London, Ontario, Canada; P. Valagussa, Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy; E. van der Wall, Afdeling Geneeskundige Oncologie, Vrije Universiteit Academisch Ziekenhuis, Amsterdam, The Netherlands.
Rights and permissions
About this article
Cite this article
Coukell, A.J., Faulds, D. Epirubicin. Drugs 53, 453–482 (1997). https://doi.org/10.2165/00003495-199753030-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199753030-00008