
Rechallenge with erlotinib in osimertinib-resistant lung adenocarcinoma mediated by driver gene loss: a case report
Introduction
Lung cancer continues to be the leading cause of cancer-related deaths worldwide (1). Treatment strategies for lung cancer have rapidly developed in recent years, particularly targeted therapy for non-small cell lung cancer (NSCLC) patients carrying driver gene mutations; for example, epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and c-ros oncogene 1 receptor kinase (ROS1) rearrangements (2). Despite initial responses to targeted therapy, there are limited treatment options available when patients inevitably develop disease progression.
Mutations of EGFR are the most common oncogenic driver in lung cancer. Targeted therapy with EGFR tyrosine kinase inhibitors (TKI) has significantly improved the overall survival and progression-free survival (PFS) in patients with lung cancer harboring EGFR activating mutations. Although some studies have focused on elucidating the mechanisms of resistance to osimertinib, methods for treatment of patients who have developed driver gene loss are inadequate (3).
Here, we report the efficacy of erlotinib re-administration in an advanced lung adenocarcinoma patient who developed driver gene loss, detected by next-generation sequencing (NGS), after developing resistance to osimertinib. We present the following case in accordance with the CARE Guideline (4).
Case presentation
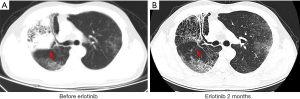
A 44-year-old man presented to hospital with cough for a month in December 2007. He did not have any specific history of medication or malignant tumor. He had a history of smoking for approximately 20 years. Computed tomography (CT) scans revealed a primary tumor located in the right lung, with bilateral pulmonary metastasis. Using CT-guided biopsy, he was diagnosed with stage IV lung adenocarcinoma with an EGFR exon 19 deletion (19del) mutation, detected using the amplification refractory mutation system (ARMS) technique (Figure 1A,B). Platinum-based chemotherapy, comprising of docetaxel plus cisplatin, was administered for two cycles. Subsequently, treatment was changed to erlotinib (150 mg per day, orally) as maintenance therapy and achieved partial response. The patient maintained a partial response for 5 years and 6 months. CT evaluation indicated an increase in lung mass size, indicating that the patient had progressive disease. Treatment was changed to a combination of pemetrexed plus endostar, with partial response for 16 months. Disease progression was evaluated after new metastatic lesions were detected in the lung. Blood and tissue samples were both sent for NGS using a panel consisting of 168 genes (LungPlasma, Burning Rock Biotech, Guangzhou, China), which revealed EGFR T790M in the tumor (Figure 1C). Treatment was then changed to osimertinib (80 mg per day, orally) and the patient achieved partial response for 26 months (Figure 1D), before disease progression, indicated by the discovery of new lung metastases (Figure 1E). Targeted sequencing of blood and tissue samples using the same 168-gene panel revealed the disappearance of both EGFR exon 19del and T790M. No other actionable mutations were detected. Erlotinib was re-administered in March, 2017 and has achieved a partial response for 26 months and he was continues to benefit from erlotinib as of this writing (Figure 1F,2). The entire treatment time line and adverse events reported by the patient during the course of the treatment are summarized in Table S1.
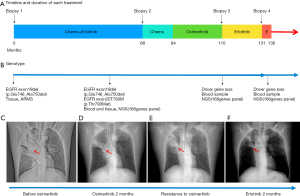
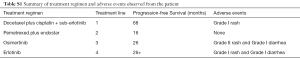
Full table
Discussion
To our knowledge, this is the first report to show the efficacy of erlotinib for treatment of a patient who developed resistance to osimertinib mediated by the loss of the oncogenic driver mutation. Our findings provide a new treatment approach for the patients in similar situation.
The discovery of EGFR TKI dramatically boosted the survival of patients with NSCLC; however, the majority developed diseases progressed with drug resistance. Osimertinib is a third-generation EGFR-TKI, targeting all EGFR sensitizing mutations including T790M mutations (5,6). The response rate to osimertinib in patients with T790M-mutant NSCLC was 61%, with a median PFS of 9.6 months (7). With the wide clinical application of osimertinib, acquired resistance has become a growing clinical challenge. Re-biopsy after osimertinib failure is an important step for guiding optimal treatment strategies for subsequent treatment lines. EGFR C797S, MET amplification, and HER2 amplification mutations are the most frequent mechanisms underlying resistance to osimertinib (8,9); however, many patients progress following osimertinib treatment, with negative ornon-actionable mutations. In our case, liquid re-biopsy and genetic analysis showed the loss of the EGFR sensitizing mutations after osimertinib failure. Despite the absence of EGFR sensitizing mutations, the re-administration of erlotinib in our patient was verified as effective and tolerable.
The EGFR-TKI, erlotinib, is an approved treatment for patients with NSCLC with activating mutations in the EGFR kinase. Erlotinib is a small molecule that can bind to the EGFR intracellular tyrosine kinase domain for ATP to block downstream signal transduction (10). In our study, NGS before treatment with erlotinib showed that EGFR was wild-type; however, the patient has achieved a partial response for 26 months at least. Re-administration of erlotinib is a potential clinical strategy to overcome osimertinib resistance; however, insufficient clinical data are available to determine its reliability. In addition, cancer cells with higher sensitivity to EGFR-TKIs may maintain their characteristics after drug resistance; hence, re-administration of erlotinib is effective.
Use of erlotinib in patients who are resistant to osimertinib and driver gene negative represents a re-challenge. Our study proves that erlotinib can be considered a potentially effective strategy for treatment of patients who develop resistance to osimertinib and are driver gene negative. The limitations with our study including the nature of work, only one case report, and lack of mechanism investigation. So, further exploration of the potential molecular mechanisms underlying this phenomenon, and large cohort studies or clinical trials, should be launched to verify the efficacy and safety of this strategy.
It is also worth mentioning that our patient had exceptional responses to all the treatments that he has received. He was diagnosed with lung cancer 11 years ago and is still benefitting from targeted therapy.
Conclusions
Overall, our case provides a clinical evidence that erlotinib is effective in overcoming osimertinib resistance despite the absence of EGFR sensitizing mutations. It also provides new treatment options for lung cancer patients with osimertinib resistance mediated by driver gene loss.
Acknowledgments
We thank the patient who participated in this study. We also thank Dr. Rui Guan for providing technical support and discussions throughout the study.
Funding: This work received financial support from the National Natural Science Foundation of Hunan Province (2018RS3106 to Yongchang Zhang, 2018JJ2238 to Yongchang Zhang and 2017SK2134 to Nong Yang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Gkolfinopoulos S, Mountzios G. Beyond EGFR and ALK: targeting rare mutations in advanced non-small cell lung cancer. Ann Transl Med 2018;6:142. [Crossref] [PubMed]
- Lettig L, Sahnane N, Pepe F, et al. EGFR T790M detection rate in lung adenocarcinomas at baseline using droplet digital PCR and validation by ultra-deep next generation sequencing. Transl Lung Cancer Res 2019;8:584-92. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Fassunke J, Müller F, Keul M, et al. Overcoming EGFR(G724S)-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun 2018;9:4655. [Crossref] [PubMed]
- Minna JD, Dowell J. Erlotinib hydrochloride. Nat Rev Drug Discov 2005.Suppl:S14-5. [PubMed]


