Current state of immunotherapy for non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer mortality worldwide and non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancers (1,2). From 2005 to 2011, 57% of the lung cancers diagnosed in the USA presented with metastases at the time of diagnosis. The prognosis for these patients with metastatic or stage IV NSCLC is extremely poor with 5-year survival rates reported as less than 5% (3). Platinum-based doublet chemotherapy is the standard first-line treatment for metastatic NSCLC when genomic testing reveals no activating epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) or ROS1 translocation/re-arrangements (found in 10–20% of NSCLC tumors) (4). Platinum-based regimens produce response rates ranging only between 15–30% (5,6). For patients whose disease progresses on first-line chemotherapy, second-line therapy historically consists of taxane-based salvage chemotherapy with a low response rate of less than 25% (7,8). In an attempt to identify novel therapeutic agents for metastatic NSCLC, multiple clinical trials have evaluated the efficacy of vaccine therapy against targets such as melanoma associated antigen 3 (MAGE-A3) and EGFR. Although these clinical trials using vaccine therapy in NSCLC provided evidence of immune stimulations with these agents, they failed to show meaningful benefits in progression-free survival (PFS) or in overall survival (OS) (9-11).
NSCLC is a remarkably heterogeneous disease that presents a large mutational load encoding a large number of potential neoantigens (12). Yet, this disease often evades the actions of the immune system. The opportunity to explore immune therapies greatly expanded with the identification of the checkpoint inhibitor agents. For the first time, these agents demonstrated responses in advanced NSCLC, with some patients exhibiting durable responses after discontinuing therapy. In 2015, two immune checkpoint inhibitors targeting programmed cell death-1 (PD-1), nivolumab and pembrolizumab were approved for second-line therapy of NSCLC (Table 1). In 2016, another checkpoint inhibitor targeting program death-ligand 1 (PD-L1), atezolizumab was recently approved for the same indication. Moreover, pembrolizumab also received approval in 2016 for first-line NSCLC treatment in patients with high PD-L1 expressing tumors. These recent approvals position immunotherapeutic agents as the preferred second line therapy for NSCLC and also have led to an increased interest in potential additional clinical applications for these agents. Immunotherapy trials in NSCLC completed so far can be broadly divided into two categories: (I) immune checkpoint inhibitors (and combinations, Table 2) and, (II) vaccine therapy.

Full table
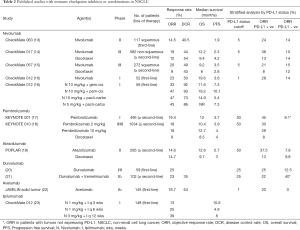
Full table
Immune checkpoint inhibition
The immune evasive measures used by cancer cells, and specifically by NSCLC cells are broadly separated into two categories defined by cellular and molecular characteristics in the tumor microenvironment: an inflamed T-cell phenotype that actively suppresses immune activation and a non-inflamed phenotype that passively escapes immune detection (24). The inflamed phenotype is characterized by tumor infiltration by CD8+ T cells which lead to the cytotoxic effect seen with T-cell response (25). As a countermeasure, tumor cells secrete cytokines such as IL-10, which promote regulatory T-cell (Treg) proliferation and suppress CD8+ T-cell-mediated cytotoxic killing (25). NSCLC tumors also have elevated expression of the chemokine CCL20, which aids in the recruitment of FOXP3+ Treg cells into the tumor microenvironment (26). Thus, Tregs play a crucial role in immune homeostasis by allowing tolerance and preventing autoimmunity through suppression of CD8+ T cells.
T-cell activation is a tightly regulated process that involves a balance between co-stimulatory and coinhibitory signals exchanged in the binding of the T-cell receptor (TCR) to the major histocompatibility complex (MHC) peptide complex or the antigen presenting cells (APCs) in addition to regulation by Treg cells described above (27). Co-inhibitory signals (i.e., immune checkpoints) serve to maintain self-tolerance and avoid destruction of normal host tissue (28). Under normal conditions, two immunologic signals are required for T-cell activation: (I) engagement of MHC-bound antigen on APCs by the TCR, and (II) co-stimulation via B7-CD28 interactions (29). The first signal generates specificity, and the latter amplifies TCR signaling, leading to T-cell activation. T-cell activation also induces a parallel, inhibitory pathway mediated by cytotoxic T-lymphocyte antigen-4 (CTLA-4) that can attenuate and terminate such responses. Many checkpoints have been described, including CTLA-4, PD-1 (and its ligand PD-L1), B7-H3, B7x, T-cell immunoglobulin, and mucin domain-containing molecule-3 (Tim-3), and B- and T-cell lymphocyte attenuator (30). To date, the best characterized and most clinically studied of these checkpoints are (I) CTLA-4 and (II) PD-1 and (III) PD-L1.
CTLA4 checkpoint inhibitors
CTLA-4 is a CD28 homolog that is expressed exclusively on T cells (31). CTLA-4 leads to downregulation of T-cell responses through several mechanisms, including outcompeting CD28 for binding of B7 molecules, inhibiting interleukin-2 (IL2) production, and preventing cell cycle progression thereby acting as a negative regulator of T-cell response.
Ipilimumab
Ipilimumab is a fully humanized monoclonal antibody (mAb) against the CTLA-4 epitope that neutralizes the receptor, thus enabling cytotoxic T cell activity and perpetuating immune responses. Most of the benefits of anti CTLA- 4 blockade seem to be mediated by depletion of Tregs as these cells demonstrate high levels of CTLA-4 expression (32). A randomized phase II trial assessed the activity of ipilimumab combined with carboplatin and paclitaxel chemotherapy in 204 untreated subjects with advanced-stage NSCLC and results were published in 2012 (33). The primary endpoint of these studies was immune-related progression-free survival (irPFS) or death, whichever occurred first. Immune-related progressive disease was defined as increase in tumor burden by 25% relative to the minimum recorded tumor burden. The patients were randomized to three groups (1:1:1) to receive paclitaxel and carboplatin with (I) placebo, (II) concurrent ipilimumab or, (III) phased ipilimumab (two doses of placebo plus paclitaxel and carboplatin followed by four doses of ipilimumab plus paclitaxel and carboplatin). The study met its primary end-point with improved irPFS in the phased ipilumumab compared to controls [hazard ratio (HR) 0.72; P=0.05]. However, similar benefit was not seen in the group receiving concurrent ipilimumab and chemotherapy. Compared to placebo, phased ipilimumab appeared to show improved efficacy for squamous histology but not for non-squamous histology. However, the study was not powered for this stratified analysis. Grade 3/4 immune-related adverse events (irAEs) were 15%, 20%, and 6% in the phased ipilimumab, concurrent ipilimumab, and the control arms, respectively. A larger phase III trial, n=1,289 (ClinicalTrials.gov, NCT01285609) is currently ongoing to compare the standard carboplatin and paclitaxel chemotherapy with concomitant administration of ipilimumab specifically in patients with squamous NSCLC. Ipilimumab is also being studied empirically in combination with targeted inhibitors (erlotinib or crizotinib) for EGFR and ALK translocation-positive NSCLC (ClinicalTrials.gov, NCT01998126), radiation (ClinicalTrials.gov, NCT02239900 & NCT02221739) and PD-1 antibody (discussed later).
Tremelimumab
Tremelimumab is also a fully human anti-CTLA4 mAb similar to ipilimumab. It failed to show an improvement in PFS in a phase II trial (n=87) that randomized patients with pretreated advanced-stage NSCLC to tremelimumab or best supportive care. The overall response rate (ORR) was only 4.8%, and no difference in PFS was reported between the two groups (34). The combination of PD-L1 antibody (durvalumab) and tremelimumab have shown better responses and is discussed in detail later.
PD-1 checkpoint inhibitors
The PD-1/PD-L1 pathway is another crucial self-tolerance pathway that tumor cells hijack to escape immune elimination. The PD-1/PD-L1 interaction inhibits T-cell response, induces apoptosis of tumor-specific T cells, and promotes differentiation of CD4 T cells into Tregs and tumor cell resistance (35). PD-1 is expressed on the surface of activated T cells, B cells, and natural killer (NK) cells (36). PD-L1 expression is reported across a range of malignancies, including NSCLC (37).
Nivolumab
Nivolumab is a fully human anti-PD-1 IgG4 mAb and is the first anti-PD-1 targeted drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of pretreated NSCLC. Results from a phase I clinical trial of BMS-936558 or Nivolumab in 296 patients with advanced cancers including NSCLC were published in 2012. Nivolumab was given at a dose of 0.1 to 10.0 mg per kilogram of body weight every 2 weeks. Patients received up to 12 cycles until disease progression or a complete response. The cumulative response rate (all doses) was 18% among NSCLC patients (14 of 76 patients); 65% of responses lasted one year or more. Grade 3/4 AEs occurred in 14% of patients; and three deaths occurred from pulmonary toxicity. Based on the clinical efficacy and safe toxicity profile compared to chemotherapy, multiple trials were initiated in NSCLC and three pivotal trials reported their results in 2015 (13-15).
The phase II, single arm CheckMate 063 trial enrolled 117 patients with advanced, refractory squamous NSCLC between November 2012 and July 2013 (ClinicalTrials.gov, NCT01721759) (13). Patients received intravenous nivolumab (3 mg/kg) every 2 weeks until disease progression or unacceptable toxic effects. A total of 14.5% (17 of 117) patients experienced an objective response, the primary endpoint for the study. Median duration of response, however, was not reached at the time of analysis. Moreover, thirty (26%) of 117 patients showed stable disease. Seventeen percent of patients reported grade 3/4 AEs; common AEs were: fatigue (4%), pneumonitis (3%), and diarrhea (3%). CheckMate 057 (ClinicalTrials.gov, NCT01673867), a randomized, open-label, international, phase III study evaluated the efficacy and safety of nivolumab compared with docetaxel in 272 patients with advanced squamous NSCLC with disease progression during or after first-line chemotherapy (15). The median OS was 9.2 months for nivolumab versus 6.0 months with docetaxel (HR 0.59; 95% CI, 0.44 to 0.79). The response rate was 20% with nivolumab versus 9% with docetaxel (P=0.008). The median PFS was 3.5 months with nivolumab versus 2.8 months with docetaxel (HR, 0.62; 95% CI, 0.47 to 0.81). The expression of the PD-1 ligand (PD-L1) was neither prognostic nor predictive of benefit.
The phase III CheckMate 017 trial (ClinicalTrials.gov, NCT01642004) enrolled 292 patients with stage IIIB/IV non-squamous NSCLC who progressed during or after first-line chemotherapy. Patients received nivolumab at 3 mg/kg every 2 weeks or docetaxel at 75 mg/m2 every 3 weeks (14). The median OS was 12.2 months (95% CI, 9.7 to 15.0) in the nivolumab group and 9.4 months (95% CI, 8.1 to 10.7) in the docetaxel group (HR, 0.73; 95% CI, 0.59 to 0.89). The response rate was 19% with nivolumab versus 12% with docetaxel (P=0.02). PFS did not favor nivolumab over docetaxel but the study was not powered for this outcome. Nivolumab was associated with even greater efficacy than docetaxel across all subgroups defined according to of tumor-membrane expression of PD-L1 (≥1%, ≥5%, and ≥10%). Serious treatment-related AEs were reported in 10% of the patients in the nivolumab group versus 54% in the docetaxel group. Based on these results, in March 2015, nivolumab was approved by the FDA for patients with previously treated advanced or metastatic NSCLC. Of note, in the CheckMate studies, PD-L1 biomarker analyses were incorporated into the study design; however, these assessments were performed retrospectively and did not factor in study eligibility.
Several ongoing clinical trials test the efficacy of nivolumab in first-line setting for advanced NSCLC. CheckMate 012, a phase I, multicohort study, explored the safety and efficacy of nivolumab as monotherapy or combined with current standard therapies in first-line advanced NSCLC (ClinicalTrials.gov, NCT01454102). Results from the monotherapy arm were published in 2016 by Gettinger et al. (16), and confirmed that the ORR was 23% (12 of 52) in patients with newly diagnosed advanced NSCLC who received nivolumab monotherapy until progression or unacceptable toxicity. The investigators also found four patients with ongoing complete responses. The ORR was 28% (9 of 32) in patients with any degree of tumor PDL 1 expression and 14% (2 of 14) in patients with no PDL 1 expression. Common AEs included fatigue (29%), rash (19%), nausea (14%), diarrhea (12%), pruritus (12%), and arthralgia (10%). Ten patients (19%) reported grade 3 to 4 treatment-related AEs. Results for first-line nivolumab plus platinum-based chemotherapy cohort (n=56) were published in September 2016 by Rizvi et al. (13). Patients received nivolumab plus chemotherapy concurrently every 3 weeks for four cycles followed by nivolumab alone until progression or unacceptable toxicity. No dose-limiting toxicities were reported. A total of 45% of patients (25 of 56 patients) reported grade 3/4 treatment-related AEs; 7% (n=4) experienced pneumonitis. This trial showed a significant rate of treatment discontinuation related to AEs with the combination at 21% (n=12) discontinued all study therapy as a result of treatment-related AEs. Objective response rates for nivolumab 10 mg/kg plus gemcitabine-cisplatin, nivolumab 10 mg/kg plus pemetrexed-cisplatin, nivolumab 10 mg/kg plus paclitaxel-carboplatin, and nivolumab 5 mg/kg plus paclitaxel-carboplatin were 33%, 47%, 47%, and 43%, respectively. Responses were achieved regardless of tumor PD-L1expression.
Rizvi et al. reported findings at the 2016 ASCO meeting for the nivolumab-ipilumumab combination cohort of CheckMate 012 (23). A total of 148 patients with advanced NSCLC received nivolumab and ipilumumab across 4 dose cohorts. Treatment-related AEs were comparable to nivolumab alone (10%), with no treatment related deaths. Across cohorts, ORRs ranged from 13% to 39%, and median duration of response was not reached. Responses were noted regardless of PD-L1 expression, with a higher magnitude of benefit in tumors that expressed PD-L1. Based on integrated data analysis, nivolumab 3 mg/kg every 2 weeks + Ipilumumab every 6 weeks was proposed for further evaluation. These results prompted a randomized phase III trial comparing the combination of nivolumab and ipilimumab (CheckMate 227; Clinical-Trials.gov, NCT02477826) against nivolumab, nivolumab plus platinum-based chemotherapy, or platinum-based chemotherapy alone in PD-L1-defined, previously untreated NSCLC.
Pembrolizumab
Pembrolizumab (MK-3475) is a high affinity humanized IgG4 mAb targeting PD-1. The efficacy and safety of pembrolizumab was assessed in NSCLC in the phase I KEYNOTE-001 study (n=495) published by Garon et al. in May 2015 (17). Patients received pembrolizumab at either 2 or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks. Common treatment-related AEs included: fatigue (19.4%), pruritus (10.7%), and decreased appetite (10.5%). Immune-mediated events were infrequent but included hypothyroidism (6.9%), pneumonitis (3.6%) and infusion-related reactions (3%). The ORR was 19.4%, and the median duration of response was 12.5 months. The median OS was 12.0 months. PD-L1 expression was evaluated using the 22C3 mAb test. On the basis of the analysis of the biopsy samples from the training group, membranous PD-L1 expression on 50% or more tumor cells (tumor proportion score, TPS ≥50%) was selected as the PD-L1 cutoff for the remainder of the trial. In total, 23.2% of patients had a TPS of ≥50% and the ORR was 45.2% in this group. Median PFS in this group was 6.3 months; median OS was not reached at the time of analysis. Thus, this study established the clinical efficacy and safe toxicity profile of pembrolizumab.
Subsequently, KEYNOTE-010 (ClinicalTrials.gov, NCT01905657), a randomized, multi-national, phase II/III study compared pembrolizumab with docetaxel in 1,034 patients with pretreated advanced NSCLC expressing PD-L1 ≥1%. Patients were randomized (1:1:1) to receive pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks. The trial used PD-L1 TPS (1–49% vs. ≥50%) as a stratification factor for randomization. The primary endpoints were OS and PFS. The median OS was 10.4 months with pembrolizumab 2 mg/kg, 12.7 months with pembrolizumab 10 mg/kg, and 8.5 months with docetaxel. OS was significantly longer for pembrolizumab 2 mg/kg versus docetaxel (HR 0.71, 95% CI 0.58–0.88) and for pembrolizumab 10 mg/kg versus docetaxel (HR 0.61, 95% CI 0.49–0.75). However, there was no significant difference in the overall median PFS. Interestingly, among patients with at least 50% of tumor cells expressing PD-L1, PFS was significantly longer with pembrolizumab compared to docetaxel. Grade 3–5 treatment-related AEs were less common with pembrolizumab than with docetaxel (13% with 2 mg/kg, 16% with 10 mg/kg, 35% with docetaxel). In an updated analysis presented at ASCO 2016, all outcomes (OS, PFS, and ORR) were reported to increase with increasing TPS with the longest OS and PFS and highest ORR in patients with TPS ≥75% (38). In October 2015, FDA approved Pembrolizumab for the treatment of patients with advanced NSCLC whose disease had progressed after chemotherapy and the tumor expresses PD-L1.
KEYNOTE-021, a phase I/II study with multiple cohorts, tested the combination of pembrolizumab with other therapeutic agents such as carboplatin, paclitaxel, bevacizumab, pemetrexed, ipilimumab, erlotinib and gefitinib (ClinicalTrials.gov, NCT02039674). The results of the dose-finding cohort of the combination of pembrolizumab with ipilimumab from KEYNOTE-021 were presented at ASCO 2016 meeting by Gubens et al. (39). As of December 2015, 45 patients received pembrolizumab 2 mg/kg and ipilimumab 1 mg/kg; 6 additional patients received the higher doses. Serious treatment-related AEs were reported in 11 (24%) patients. Four (9%) patients discontinued treatment because of treatment-related AEs. ORR was 24% (2 complete responses); 18 (40%) patients had SD. There was no link between PD-L1 status and outcome. Therefore, combination of pembrolizumab and ipilumumab demonstrated a significant toxicity profile and an ORR similar to that of pembrolizumab alone. Results from the cohorts A–C of KEYNOTE-021 were also reported in ASCO 2016 meeting. Chemotherapy-naive, advanced NSCLC patients were randomly assigned to pembrolizumab 2 or 10 mg/kg every 3 weeks plus carboplatin and paclitaxel (A, any histology); carboplatin, paclitaxel and bevacizumab (B, nonsquamous); or carboplatin and pemetrexed (C, nonsquamous) for 4 cycles followed by maintenance with pembrolizumab and bevacizumab for (B) and pemetrexed for (C). As of December 2015, 74 patients have been treated. One DLT occurred in cycle 1 (grade 3 rash, C). Grade 3/4 treatment-related AEs occurred in 36%, 46%, and 42% of patients, in cohorts A, B, and C, respectively, most commonly AST elevation (n=3 in C), anemia (n=2 each in A and C), and febrile neutropenia (n=2 each in A and B). ORR was 57% on combining all cohorts and highest for cohort C at 71% (39).
Pembrolizumab was tested as first line therapy for metastatic treatment-naive NSCLC compared to different chemotherapy regimens in the phase III, KEYNOTE-024 trial (ClinicalTrials.gov, NCT02142738). In this trial, 154 patients with previously untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells and no sensitizing EGFR mutation or ALK rearrangement received pembrolizumab and 151 received the investigator’s choice of platinum-based chemotherapy. Crossover from the chemotherapy group to the pembrolizumab group was permitted in the event of disease progression. The primary end point, PFS was significantly longer in the pembrolizumab group compared to chemotherapy group (10.3 vs. 6.0 months; HR 0.50; P<0.001). The OS was also significantly better in the pembrolizumab group vs. the chemotherapy group (HR 0.60; 95% CI, 0.41 to 0.89; P=0.005). The response rate was 44.8% in the pembrolizumab group and 27.8% in the chemotherapy group. Severe AEs occurred in only 26.6% of the patients in the pembrolizumab group compared to 56.6% in the chemotherapy group. Based on the significant improvement in PFS and OS reported by this study, FDA approved pembrolizumab for the first-line treatment of patients with metastatic NSCLC whose tumors express PD-L1 on at least 50% of tumor cells (40). A current trial also evaluates the activity of pembrolizumab in untreated NSCLC patients with brain metastases (ClinicalTrials.gov, NCT02085070).
PD-L1 checkpoint inhibitors
While PD-1 antibodies target the PD-1 receptor on activated immune cells, PD-L1 inhibitors block the interaction between PD-L1 and PD-1 and the interaction between PD-L1 and B7.1 (an inhibitory receptor on T cells).
Durvalumab
Durvalumab (MEDI-4736) is a selective, high-affinity, human immunoglobulin G1 κ anti-PD-L1 mAb. A phase I/II trial evaluated the safety and efficacy of durvalumab in advanced NSCLC and other solid tumors. Durvalumab 10 mg/kg was given every 2 weeks for up to 12 months to treatment-naïve patients with Stage IIIB/IV NSCLC. Fifteen patients were initially enrolled regardless of PD-L1 status. After a protocol amendment, enrollment was restricted to patients with PD-L1 positive tumors. As of Dec 2015, 59 patients (48 PD-L1 positive) received a median of 8 doses (range, 1–27 doses). Grade ≥3 drug-related AEs were reported in 9% of patients and led to discontinuation in 4 patients (7%); most frequent was diarrhea (2 patients). In the 52 evaluable patients with ≥12 weeks of follow-up, ORR was 25% and disease control rate was 56% (24/43 PD-L1+; 4/8 PD-L1). ORR was similar for all histologies (20). Based on these promising results, multiple trials now evaluate the efficacy of MEDI-4736 as monotherapy (ClinicalTrials.gov, NCT02087423), after concurrent chemoradiotherapy in stage III (ClinicalTrials.gov, NCT02125461) and as adjuvant therapy in stage IB to IIIA NSCLC (BR31 trial; ClinicalTrials.gov, NCT02273375).
While PD-L1/PD-1 inhibitors prevent inhibition of T-cell function, CTLA-4 pathway inhibitors expand the number and repertoire of tumor-reactive T cells. In order to test the hypothesis that combining PD-1/PD-L1 inhibition with CTLA-4 inhibition may show synergistic effect; an open-label, phase Ib study was conducted in immunotherapy-naïve patients with confirmed advanced NSCLC. In the dose-escalation phase, patients received durvalumab in doses of 3, 10, 15, or 20 mg/kg every 4 weeks, or 10 mg/kg every 2 weeks, and tremelimumab in doses of 1, 3, or 10 mg/kg every 4 weeks for 6 doses then every 12 weeks for 3 doses. A total of 102 patients were enrolled by April 1, 2015. Treatment-related serious AEs occurred in 36% of patients with the most frequent being diarrhea (11%), colitis (9%), and increased lipase (8%). Discontinuations attributable to treatment-related AEs occurred in 28% of patients. Twenty-two patients died during the study, of which three deaths were related to treatment. Investigator-reported confirmed ORR were achieved by 6 (23%) of 26 patients in the combined tremelimumab 1 mg/kg cohort. Clinical activity was noted irrespective of PD-L1 status (21). Based on the available safety and clinical data, durvalumab 20 mg/kg every 4 weeks plus tremelimumab 1 mg/kg every 4 weeks was selected for dose expansion. With these encouraging results, multiple phase II/III studies have been launched in NSCLC using this combination of immunotherapy agents, including the third-line ARCTIC (ClinicalTrials.gov, NCT02352948) & first-line MYSTIC (ClinicalTrials.gov, NCT02453282) and NEPTUNE (ClinicalTrials.gov, NCT02542293).
Atezolizumab
Atezolizumab (MPDL3280A) is a fully humanized, engineered IgG1 mAb against PD-L1. A phase I study evaluated the safety and activity of MPDL3280A in advanced solid tumors. In this study, the drug was well tolerated with only 12.6% of patients experiencing grade 3–4 AEs, the most common of which was fatigue. Clinical efficacy was observed across all tumor types with ORR of 21% (n=175) and 23% in NSCLC (n=53). For NSCLC, higher ORR was seen in tumors expressing PD-L1 by IHC (41). Subsequently, a single arm phase II study of atezolizumab (BIRCH) in PD-L1 selected advanced NSCLC was initiated. The primary objective of this trial was ORR and 667 patients with PD-L1 expressing tumors (higher IHC level of TC2/3 or IC2/3 as determined by SP142 assay) were enrolled. Besse et al. presented the preliminary results at the European Cancer Congress in 2015. As of May 2015 (data cutoff), BIRCH met its primary endpoint in all predefined subgroups with ORR ranging from 17% to 27%. Eleven percent of patients had a treatment-related Grade 3/4 AE, the most common being fatigue (18%) and nausea (10%). Six percent of patients discontinued study treatment due to an AE. BIRCH demonstrated clinically meaningful efficacy as monotherapy in patients with PD-L1-selected advanced NSCLC, with no unexpected toxicities.
The randomized phase II POPLAR trial (ClinicalTrials.gov, NCT01903993) evaluated the efficacy and safety of atezolizumab versus docetaxel in previously treated NSCLC. Patients were stratified by PD-L1 tumor-infiltrating immune cell status, histology, and previous lines of therapy, and randomly assigned (1:1) to receive intravenous atezolizumab 1,200 mg or docetaxel 75 mg/m2 every 3 weeks. The primary endpoint was OS. OS was 12.6 months for atezolizumab versus 9.7 months for docetaxel (HR 0.73, P=0.04). Increasing improvement in OS was associated with increasing PD-L1 expression. Eleven percent of the patients in the atezolizumab group versus 39% in the docetaxel group experienced treatment-related grade 3/4 AEs. This trial showed that Atezolizumab significantly improved survival compared with docetaxel in patients with previously treated NSCLC (19). Based on the results of the POPLAR and BIRCH studies, FDA granted approval to atezolizumab for patients with advanced NSCLC whose disease progressed on platinum-based chemotherapy in 2016. There are now ongoing studies of atezolizumab combined with chemotherapy (ClinicalTrials.gov, NCT02657434, NCT02409342, NCT02367781, NCT02366143) and as monotherapy for PD-L1 positive treatment naïve advanced NSCLC (ClinicalTrials.gov, NCT01846416). Moreover, the efficacy of combination treatments with erlotinib/alectinib (ClinicalTrials.gov, NCT02013219) and the MEK inhibitor cobimetinib (ClinicalTrials.gov, NCT01988896) are also being evaluated.
Avelumab
Avelumab (MSB0010718C) is a fully human anti-PD-L1 IgG1 mAb. A phase I dose-escalation trial evaluated the safety and clinical activity of avelumab in metastatic solid tumors (JAVELIN Solid Tumor; ClinicalTrials.gov, NCT01772004). Dose expansion in multiple cohorts including untreated NSCLC as well as NSCLC pretreated with platinum based chemotherapy. Results from the previously treated NSCLC cohort were reported at ASCO 2015. This study enrolled 184 patients who received avelumab, and ORR was 12% and stable disease reported in 38%. Median PFS was 11.6 weeks (3). This led to an ongoing phase III trial to assess efficacy and safety of avelumab compared with docetaxel in recurrent NSCLC (JAVELIN LUNG 200; ClinicalTrials.gov, NCT 02395172). Results from the untreated first-line NSCLC cohort were presented at the ASCO annual meeting in 2016 by Verschraegen et al. Patients with untreated advanced NSCLC (n=145) not selected for PD-L1 expression were treated with avelumab 10 mg/kg IV every 2 weeks. Common treatment-related AEs were infusion-related reactions (16.6%) and fatigue (14.5%). Unconfirmed ORR was 18.7% in 75 patients and disease control rate was 64.0%. ORR was 20% in PD-L1 positive patients based on a ≥1% cutoff for tumor cell staining (35 out of 45 patients). Single-agent avelumab showed an acceptable safety profile and clinical activity in NSCLC not previously treated for advanced disease and unselected for PD-L1 expression (22).
Immune-related toxicities of immune checkpoint inhibitors
Although anti-PD-1 and anti-PD-L1 antibodies show significant clinical benefits, they can lead to significant inflammatory side effects called irAEs by increasing immune system function. These autoimmune side effects are less frequent and less severe than the toxicities observed with chemotherapy, but require management with anti-inflammatory drugs such as steroids or infliximab in patients who develop these toxicities. The most common immune related side effects involve the endocrine system (hypophysitis, <1% and hypothyroidism, 1–7%), skin (rash, 4–13% and infusion-related reactions, 2–3%), gastrointestinal tract (diarrhea, 6–12% and colitis, <1%), lung (pneumonitis, 3–5%), liver (hepatitis, <1%), and kidneys (renal insufficiency, <1%) (14,15,17-20,22). Significant patient education and vigilant oversight are needed to address these auto immune-related toxicities quickly to avoid development of severe symptoms. So far, no correlation is suggested between the occurrence of an immune-mediated AE and long-term outcomes to immune checkpoint-blocking antibody therapy (42). The common AEs noted with these agents are listed in Table 3.
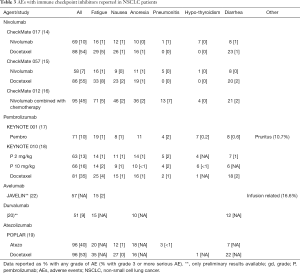
Full table
Assessment of tumor response to immunotherapy
Resulting clinical response patterns with immunotherapy can extend beyond those of cytotoxic agents and manifest as an initial slight increase in tumor size due to lymphocyte infiltration and inflammatory edema. Response evaluation criteria in solid tumors (RECIST) designed to detect effects of cytotoxic agents, may not provide a complete assessment of immunotherapeutic agents (43,44). Immune-related response criteria (irRC) were therefore developed to provide more specific characterization of the atypical response patterns observed in the phase II development program for ipilimumab in melanoma. Initial evidence of disease progression is handled differently with irRC compared with conventional response criteria. For example, irRC require confirmation of initial evidence of progressive disease, whereas RECIST do not. Similarly, appearance of new lesions would define progression of disease by RECIST; whereas, with irRC, new lesions may be added to the sum of the products of the two largest perpendicular diameters of all index lesions at any time point and will only result in progressive disease if the sum is ≥25% compared with nadir (43).
Biomarkers for immune checkpoint inhibition
Given the limited response, considerable efforts have been invested to develop biomarkers to predict which patients should receive immune checkpoint inhibitors.
PD-1 expression
The most widely studied biomarker is PD-L1 expression on tumor cells especially as multiple studies suggested better responses in PD-L1 expressing tumors. But the presence of robust responses in some patients with no or low levels of PD-L1 expression complicates the use of PD-L1 as an exclusionary predictive biomarker. Two commercial complementary PD-L1 diagnostic tests for nivolumab (Dako 28-8 pharmDx) and pembrolizumab (Dako 22C3 pharmDx) are now FDA approved for use in NSCLC, while the complementary test for atezolizumab (Ventana SP142) is approved for urothelial carcinoma. The performance characteristics of the companion tests for atezolizumab and durvalumab (Ventana SP263) are still being assessed in NSCLC (45). Despite the development and approval of these commercial IHC assays, the use of PD-L1 IHC as a predictive biomarker is confounded by multiple unresolved issues which include differing IHC cutoffs, tissue preparation, processing variability, primary versus metastatic biopsies, oncogenic versus induced PD-L1 expression, intratumor heterogeneity, staining of tumor versus immune cells and the subjective nature of the assessment (46,47). PD-L1 expression may also be affected by concurrent or prior treatments, including radiation or chemotherapy, which was likely administered after a biopsy was obtained (48,49). A consortium of drug manufacturers and representatives from Dako and Ventana, organized in part through a joint effort of the FDA, AACR, ASCO, and the International Association for the Study of Lung Cancer (IASLC), was formed with the task of creating a resource to compare the performances of the four major PD-L1 companion assays. Results of the pilot phase of this “Blueprint project” were presented at the AACR Annual Meeting in 2016 by Ratcliffe et al., and included 81 lung cases reviewed by three pathologists. Tumor cell PD-L1 staining was similar for the Ventana SP263 and the two Dako assays, but with less tumor staining by the Ventana SP142 assay. The final results from this study are eagerly awaited.
Genomic instability
NSCLC is a tumor associated with increased genomic instability. The mutations underlying genomic instability have the potential to generate tumor-specific antigens. Rizvi et al. recently demonstrated through whole-exome sequencing in tissue from NSCLC patients treated with pembrolizumab, that higher non-synonymous mutation burden in tumors is associated with improved objective response and PFS (12). This observation is consistent with the hypothesis that the efficacy of anti-PD-1 therapy is largely related to recognition of neoantigens; i.e., those resulting from various somatic mutations induced by carcinogens. In this study, efficacy of therapy positively correlated with the molecular smoking signature (tobacco-related mutagenesis), higher neoantigen burden (correlated with higher mutational burden), and mutations in certain DNA repair pathways. Also, in the KEYNOTE-001 trial, the response rate to pembrolizumab was 22.5% for current/former smokers compared to 10.3% in never smokers (17), further supporting that the higher mutational burden associated with smoking contributed to improved response to PD-1 inhibition.
Others
Tumeh et al. demonstrated that CD8 tumor-infiltrating lymphocytes or TILs in the tumor microenvironment are associated with increased responsiveness to PD-1 inhibition (43). Presence of EGFR mutations and ALK rearrangements (alterations typically associated with a lack of tobacco exposure) were suggested as associated with lower ORR to PD-1 inhibitors (50). Therefore, trials are ongoing to test if ORR to immunotherapy can be improved in these tumors if given concurrent TKI therapy.
Vaccine therapy in NSCLC
Vaccine therapy aims to boost immune response against tumor cells using patient’s own immune-surveillance mechanism. This is achieved through the administration of immunogenic tumor-associated antigens or cells in conjunction with an immunoadjuvant that elicits specific antitumor immune response by the patient’s own immune system. Many of the vaccine trials in NSCLC showed an immune response after vaccination as discussed below, usually in the form of an increase of target specific cytotoxic T-cells. Unfortunately, this has not translated into significant survival advantages in the phase III trials to date. In terms of toxicity, most of these vaccine-based therapies show less toxicity when compared to traditional chemotherapies or other immune therapies. In our discussion about vaccine trials for NSCLC, we divided the vaccines into four different general categories by the methodological approach used to produce the vaccine.
Antigenic target vaccines
These therapeutic vaccines identify specific tumor-associated antigen and aim at eliciting targeted anti-tumor cellular immune responses and are often combined with an adjuvant to strengthen the immune response.
MAGE-A3
MAGE-A3 is a tumor-specific protein that promotes survival of malignant cells (51) and is expressed on 39.2% of lung tumors (52). A randomized, double-blind, placebo-controlled trial (MAGRIT), evaluated the MAGE-A3 immunotherapeutic (recombinant MAGE-A3 with AS15 immunostimulant) compared to placebo in 2,312 completely resected Stage IB, II and IIIA NSCLC patients with MAGE-A3 expressing tumors (ClinicalTrials.gov, NCT00480025). In March 2014, Glaxo Smith-Kline announced that the trial did not meet its primary endpoints of DFS. Subsequently, in April, 2014, the MAGRIT trial was completely closed and the agent was abandoned.
EGFR
A new vaccine contains humanized recombinant EGF antigen attached to an adjuvant carrier protein as well as low-dose cyclophosphamide as an immunoadjuvant agent and targets EGFR pathway. In the phase 1 trial for this vaccine, the vaccine was administered before and after standard first-line chemotherapy in advanced NSCLC to 20 patients. Ninety-two percent of the evaluated patients (n=13) showed an immune-dominant antibody response against the central region on the EGF molecule (53). An international phase III trial was started in September of 2014 to determine whether the recombinant human EGF cancer vaccine is safe and effective in the treatment of stage IV NSCLC in patients with a serum EGF concentration of >250 pg/mL (ClinicalTrials.gov, NCT02187367).
Mucin 1, cell surface associated (MUC1)
Tecemotide is a synthetic lipopeptide derived from MUC-1, with Monophosphoryl lipid A as an adjuvant (54). A phase I study showed the safety and immunogenicity of this vaccine in stage IIIB or IV NSCLC. Immunological assays revealed the generation of cytotoxic T lymphocytes against MUC1-positive tumor cell lines in 5 of 12 evaluable patients. The vaccine appeared to be well tolerated (55). A randomized double-blind phase III trial (START) then started to investigate whether the MUC1 antigen-specific vaccine tecemotide improved survival in patients with stage III unresectable NSCLC when given as maintenance therapy after chemoradiation (concurrent or sequential) compared to placebo. From February 2007 to November 2011, 1,513 patients were randomly assigned to tecemotide or to placebo). Median OS was 25.6 months with tecemotide vs. 22.3 months with placebo (P=0.123). However, there was a significant survival benefit in subgroup of patients who initially received concurrent chemoradiotherapy (30.8 vs. 20.6 months, P=0.016) (56). This data led to a multi-center phase III trial (START2 trial) in unresectable stage III NSCLC. However, the sponsor decided to discontinue the program with Tecemotide in NSCLC and terminated the study early as a phase I/II study of tecemotide in Japanese patients with stage III NSCLC did not show a benefit in OS or any of the secondary endpoints (11,57).
Telomerase
Vaccines that target hTERT (Telomerase reverse transcriptase) elicit a combined form of helper-T cell and cytotoxic-T cell response. A phase I/II study (CTN-2000) investigated the safety, tolerability and clinical response to vaccination with a combination of telomerase peptides GV1001 (hTERT: 611–626) and HR2822 (hTERT: 540–548) in 26 patients with NSCLC. The treatment was well tolerated and immune responses against GV1001 were detected in 11 of 24 patients (58). On long-term follow-up, immune responders achieved an increased survival compared with non-responders (19 vs. 3.5 months; P<0.001). This led to a phase II trial (CTN-2006) in inoperable stage III NSCLC patients treated with chemo-radiation followed by GV1001 vaccination. GV1001-specific immune response developed in 16/20 evaluable patients. Long-term immune-monitoring showed persisting responses in 13 subjects (59). The considerable immune response rate and low toxicity in this trial supported the concept of combining chemo-radiation with vaccination. A phase III trial thus started in inoperable stage III NSCLC to evaluate survival in patients receiving vaccination with GV1001 following chemo-radiation (ClinicalTrials.gov, NCT01579188).
Whole cell vaccines
These therapeutic vaccines are developed from autologous or allogenic tumor cells with the aim of eliciting immunity against a broad spectrum of tumor-associated antigens. Whole cell vaccines are usually genetically modified to express cytokines, chemokines or costimulatory molecules to stimulate the immune response to the injected irradiated tumor cells.
GVAX
GVAX is a single cell preparation of autologous tumor collected from the patient and infected with an adenoviral vector encoding GM-CSF. At the IASLC meeting in 2007, Davies and colleagues presented the data from the SWOG 0310 trial of GVAX in patients with advanced bronchoalveolar cancer (9). Only nine of 18 patients eventually received vaccination due to multiple issues (inadequate vaccine production, progressive disease, death, erroneous pathology). No responses were seen in treated patients. The interest in developing the GVAX vaccine in NSCLC waned due to the modest clinical activity, and the significant logistical barriers of vaccine production.
Belagenpumatucel-L
Belagenpumatucel-L is a an allogeneic whole tumour cell vaccine comprised of four NSCLC cell lines transfected with a human transforming growth factor (TGF)-β2-antisense vector designated pCHEK/HBA2. A phase II study of Belagenpumatucel-L in patients with stage II-IV NSCLC showed a response rate of 15% in 75 patients who received the vaccine therapy. A 47% 2-year survival was seen in patients on the high dose cohorts (n=41) (60). A subsequent trial was performed with a higher dose of 2.5×107 cells in patients with advanced pretreated NSCLC who received monthly vaccinations for 16 months. Seventy percent of patients showed no evidence of disease progression at week 16 (61). Given these interesting results, a phase III study (STOP) enrolled 532 patients with Stage III/IV NSCLC who did not progress after platinum-based chemotherapy. There was no difference in survival between the groups (HR 0.94; P=0.594) or in PFS. Although the overall trial did not meet its survival endpoint, improved survival for belagenpumatucel-L was suggested in patients who were randomized within 12 weeks of completion of chemotherapy (10). The FDA expressed interest in continuing to investigate this immunotherapeutic in the subsets of patients that derived a benefit in this study.
Tergenpumatucel-L
Tergenpumatucel-L consists of allogenic lung cancer cells from three NSCLC cell lines that have been genetically modified to express the carbohydrate alpha-1,3-galactosyltransferase {α[1,3]Gal}, to which humans have an inherent pre-existing immunity. A phase II trial, presented at the ASCO meeting in 2013 included 28 patients with advanced, previously treated NSCLC and reported no serious AEs with the administration of this agent (62). Eleven out of 18 patients had a greater than tenfold increase in interferon gamma after treatment, and this finding suggested a positive correlation with improved survival. As an unexpected finding, 31% of patients who progressed after treatment achieved a response to further chemotherapy while 25% of patients achieved stable disease. This finding suggested that tergenpumatucel-L might have a potential chemo sensitizing effect, and led to a phase IIB/III trial (ClinicalTrials.gov, NCT01774578).
Vector based vaccines
These therapeutic vaccines utilize a vector such as an attenuated virus to deliver vaccine to the antigen-presenting cells with the aim of inducing an immune response.
TG4010
TG4010 (MVA-MUC1-IL2) is a suspension of recombinant vaccinia virus of the Ankara strain (MVA) that expresses the coding sequences of the MUC1 antigen and IL2. A phase II study randomized 65 chemotherapy-naïve patients with stage IIIB/IV NSCLC to chemotherapy with cisplatin/gemcitabine with concurrent MVA-MUC1-IL2 or MVA-MUC1-IL2 alone (63). In the combination arm, 13/44 patients achieved a partial response. In the MVA-MUC1-IL2 alone arm, no responses were seen. This trial suggests that co-administration of MVA-MUC1-IL2 with chemotherapy is safe, and does not appear to hamper efficacy. Another randomized phase II study compared the same chemotherapy regimen with or without MVA-MUC1-IL2 in 148 chemotherapy naïve patients with stage IIIB/IV NSCLC (64). Six month PFS, the study’s primary endpoint, was not statistically different. An exploratory analysis suggested that patients with the NK cells phenotype normal group had a better PFS with combination therapy, suggesting a significant regulatory role for NK cells in the adaptive immune response. This study that started in 2012 with a planned completion date in 2019 is currently recruiting patients in the phase III portion (ClinicalTrials.gov, NCT01383148).
Idiotype-based
This approach uses active specific immunotherapy to generate an effective immune response against tumor-associated antigens using anti-idiotype monoclonal antibodies. Racotumomab-alum is an anti-idiotype vaccine targeting the N-glycosylated tumor-associated ganglioside (NeuGcGM3) (65). In a randomized multicenter placebo-controlled phase II/III trial, 166 patients with stage IIIb/IV NSCLC and at least stable disease after first-line chemotherapy were randomized to the vaccine or placebo. Median OS in the two groups was 8.23 and 6.80 months, respectively (P=0.004; P=0.004). Although the difference in survival was significant between the two groups, both median survivals were shorter compared to historical controls and the study included participants only from Cuba (66). A randomized, open label, multicenter phase III trial thus started to evaluate the efficacy and safety of racotumomab versus best supportive care in patients with advanced NSCLC who achieved disease control with standard first-line treatment (ClinicalTrials.gov, NCT01460472).
Conclusions and future directions
Immunotherapy for NSCLC has recently evolved into a true treatment modality with the acceptance of PD-1 and PD-L1 inhibitors as the new standard of care for second-line treatment of NSCLC. While two PD-1 inhibitors (Nivolumab and Pembrolizumab) and one PD-L1 inhibitor (Atezolizumab) have been approved by the FDA for NSCLC, other inhibitors of PD-1/PD-L1 pathway are also being developed at a rapid pace. The PD-1 inhibitor, pembrolizumab has also been approved for the first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression (≥50% of tumor cells). However, it is still at the discretion of the treating physician whether to use PD-1 or PD-L1 inhibitor for second-line NSCLC therapy as data to compare these two pathways is lacking. Also, with the establishment of these agents for metastatic NSCLC, focus is now on exploring their role in the adjuvant as well as consolidation setting for NSCLC. Moreover, although these agents have shown tremendous potential, response rates still remain low except for tumors expressing PD-L1 at high levels. Therefore, significant efforts are being put into evaluating novel combinations combining PD-1 or PD-L1 inhibitors with other agents such as chemotherapy, radiation and other immunotherapeutic agents. The optimal duration of immunotherapy in patients with durable responses is also another unanswered question. While vaccine therapy trials have so far failed to show significant clinical benefit, the demonstration of enhanced immune response in these trials suggest the vaccine therapy needs additional evaluation in combination with other therapeutic modalities especially checkpoint inhibition. While the PD-1 and PD-L1 inhibitors have been received accelerated FDA approvals, the development of predictive and prognostic biomarkers for these agents have lagged far behind and remains a crucial area for future research.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Gulley JL, Spigel D, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33:suppl; abstr 8034.
- Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol 2012;138:332-46. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Kelly K, Crowley J, Bunn PA Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 2001;19:3210-8. [Crossref] [PubMed]
- Weiss JM, Stinchcombe TE. Second-Line Therapy for Advanced NSCLC. Oncologist 2013;18:947-53. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Davies AM, Mudad R, Moon J, et al. Phase II trial of autologous cancer vaccine, CG8123 (GVAX), in patients with advanced bronchioloalveolar carcinoma (BAC): a Southwest Oncology Group Study. J Thorac Oncol 2007;2:S461. [Crossref]
- Giaccone G, Bazhenova LA, Nemunaitis J, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 2015;51:2321-9. [Crossref] [PubMed]
- Nokihara H, Katakami N, Hida T, et al. Phase I/II study of tecemotide cancer immunotherapy for Japanese patients with unresectable stage III non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33;abstr 3036.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Antonia SJ, Km SW, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non-small-cell lung cancer. J Clin Oncol 2016;34;abstr 9029.
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Verschraegen CF, Chen F, Spigel DR, et al. Avelumab (MSB0010718C; anti-PD-L1) as a first-line treatment for patients with advanced NSCLC from the JAVELIN Solid Tumor phase 1b trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34;abstr 9036.
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Neurath MF, Finotto S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine Growth Factor Rev 2012;23:315-22. [Crossref] [PubMed]
- Zhang CY, Qi Y, Li XN, et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed Pharmacother 2015;69:242-8. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 2008;224:166-82. [Crossref] [PubMed]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515-48. [Crossref] [PubMed]
- Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther 2014;96:214-23. [Crossref] [PubMed]
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987;328:267-70. [Crossref] [PubMed]
- Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009;206:1717-25. [Crossref] [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [Crossref] [PubMed]
- Zatloukal P, Heo DS, Park K, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2009;27;abstr 8071.
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381-8. [Crossref] [PubMed]
- Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765-72. [Crossref] [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [Crossref] [PubMed]
- Baas P, Garon EB, Herbst RS, et al. Relationship between level of PD-L1 expression and outcomes in the KEYNOTE-010 study of pembrolizumab vs docetaxel for previously treated, PD-L1–Positive NSCLC. J Clin Oncol 2016;34;abstr 9015.
- Gubens MA, Sequist LV, Stevenson J, et al. Phase I/II study of pembrolizumab (pembro) plus ipilimumab (ipi) as second-line therapy for NSCLC: KEYNOTE-021 cohorts D and H. J Clin Oncol 2016;34:suppl; abstr 9027.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015.76-83. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297-306. [Crossref] [PubMed]
- Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer 2016;4:48. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 2016;6:20090. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Atanackovic D, Hildebrandt Y, Jadczak A, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica 2010;95:785-93. [Crossref] [PubMed]
- Sienel W, Varwerk C, Linder A, et al. Melanoma associated antigen (MAGE)-A3 expression in Stages I and II non-small cell lung cancer: results of a multi-center study. Eur J Cardiothorac Surg 2004;25:131-4. [Crossref] [PubMed]
- Neninger E, Verdecia BG, Crombet T, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother 2009;32:92-9. [Crossref] [PubMed]
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res 2007;13:s4652-4. [Crossref] [PubMed]
- Palmer M, Parker J, Modi S, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer 2001;3:49-57;discussion 8. [Crossref] [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Berman AT, Simone CB 2nd. Immunotherapy in locally-advanced non-small cell lung cancer: releasing the brakes on consolidation? Transl Lung Cancer Res 2016;5:138-42. [PubMed]
- Brunsvig PF, Aamdal S, Gjertsen MK, et al. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother 2006;55:1553-64. [Crossref] [PubMed]
- Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res 2011;17:6847-57. [Crossref] [PubMed]
- Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30. [Crossref] [PubMed]
- Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009;16:620-4. [Crossref] [PubMed]
- Morris JC, Rossi GR, Harold N, et al. Potential chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31;abstr 8094.
- Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol 2008;3:735-44. [Crossref] [PubMed]
- Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011;12:1125-33. [Crossref] [PubMed]
- Vázquez AM, Hernandez AM, Macias A, et al. Racotumomab: an anti-idiotype vaccine related to N-glycolyl-containing gangliosides - preclinical and clinical data. Front Oncol 2012;2:150. [Crossref] [PubMed]
- Alfonso S, Valdes-Zayas A, Santiesteban ER, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 2014;20:3660-71. [Crossref] [PubMed]


