-
PDF
- Split View
-
Views
-
Cite
Cite
Benedetto Ielpo, Andres Sanchez Pernaute, Stefano Elia, Oreste Claudio Buonomo, Luis Diez Valladares, Elia Perez Aguirre, Giuseppe Petrella, Antonio Torres Garcia, Impact of number and site of lymph node invasion on survival of adenocarcinoma of esophagogastric junction, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 5, May 2010, Pages 704–708, https://doi.org/10.1510/icvts.2009.222778
Close - Share Icon Share
Abstract
Lymph node involvement in adenocarcinoma of the esophagogastric junction (EGJ) is similar to that of gastric cancer. The impact on survival of the number and site of lymph nodes involved in a subgroup of patients undergone surgery for adenocarcinoma of EGJ is reported. Sixty-four patients undergone transthoracic esophagectomy with two-field lymphadenectomy for adenocarcinoma of the EGJ were retrospectively assessed. Five-year survival according to AJCC gastric cancer nodal classification and central node invasion was evaluated. In N0 patients survival was assessed in relation to the number of lymph nodes removed. Five-year survival was 72% in N0, 46% in N1 and 0% in N2 and N3 group. Intergroup differences were statistically significant (P<0.05) except between N2 and N3 groups. Overall survival was different depending on the infiltration of distal or proximal site nodes, 23% vs. 58% (P<0.05); in N0 patients it was related to the number of lymph nodes removed (83% >15 vs. 57% <15, P<0.05). Classification of lymph node involvement in adenocarcinoma of the EGJ by gastric cancer criteria is adequate for prognostic purposes. The involvement of distal nodes in all cases and the removal of <15 nodes in N0 group resulted as independent negative predictive factors.
1. Introduction
The incidence of adenocarcinoma of the esophagus has significantly increased since the mid-1970s until replacing squamous cell carcinoma as the most common esophageal tumor [1]. This tumor is located mainly in the distal esophagus, namely the esophagogastric junction (EGJ). This area was described by Siewert at 5 cm above and below the upper end of the gastric mucosa folds (endoscopic cardia) who also identified three types of adenocarcinoma depending on its location: type I, –5 to –2 cm from the true cardia; type II, –1 to +2 cm; type III +2 to +5 cm from the EGJ [2].
Adequate lymphadenectomy has been often advocated since the presence of any lymph node metastases is associated with poor prognosis compared with node-negative patients.
Recently, the distribution of lymph node metastases in adenocarcinoma of the EGJ has been shown to have a different pattern compared with other esophageal tumors [3–7] so that it is now considered as distinct from esophageal or gastric cancer. Moreover, the prognostic value of the number and location of positive nodes in adenocarcinoma of the EGJ has been emphasized [4, 6, 7].
In the current TNM classification for all esophageal cancers only metastatic lymph nodes are taken into account (pN0: no involved nodes; pN1: nodal involvement). Since variations in N stage distribution between treatment arms of a trial significantly impact on the outcome, a greater reliability in EGJ cancer staging is warranted. A lymph node classification considering both the number and location of metastatic nodes may add relevant prognostics.
Therefore, in our study, we compared the current esophageal cancer staging with a new one that considers the number and location of positive lymph nodes to establish which provides a better prognostic stratification for EGJ adenocarcinoma.
2. Materials and methods
During a period of six years (January 2002–December 2008), 137 patients underwent esophagectomy for malignancy in the Department of General Surgery 2 at Clinico San Carlos Hospital in Madrid; among them, 84 patients were affected by adenocarcinoma of the EGJ.
All patients undergone esophagectomy with curative intent and an histologically proven R0 adenocarcinoma located in the EGJ were included while those with R1 and R2 resection were excluded as well as patients who died during hospitalization or within the 30th postoperative day, as their deaths were considered not directly related to tumor staging. Data recorded were patient demographics, tumor differentiation, T and M stage, number and location of analyzed lymph nodes. Time to death was obtained from the ambulatory medical records.
All these patients underwent preoperative CT-scan and endoscopy and were classified as Siewert type I and II since in reporting institution type III is considered as a gastric cancer in which a standard gastrectomy without chest lymphadenectomy is usually performed.
No neoadjuvant therapy was administered. Those patients who were postoperatively proven to have positive nodes or T4 underwent adjuvant chemotherapy. No radiotherapy was performed. Surgical procedure was a subtotal Ivor–Lewis esophagectomy with proximal gastric resection through a right thoracotomy and two-field lymphadenectomy in all cases.
In the abdominal step, dissection of lymph nodes was performed along the cardia, lesser and large curvature, left gastric artery, celiac axis, common hepatic artery, and splenic artery, as D2 in gastric cancer, marking the location of the lymph node on the specimen at the time of surgery. Mediastinal anastomosis was performed by mechanical gastric tubulization.
Nodes from the cardia, lesser and large curvature and left gastric artery were included in the proximal node group; distal node group included those from the celiac axis, common hepatic artery, lower mediastinum and tracheal bifurcation.
The circumferential resection margin was considered free if a microscopic evidence of a tumor was at least 1 mm away from the edge.
Statistical analysis was performed by means of the SPSS® program (Version 15.0; Chicago, IL, USA). Survival was assessed until January 2009 by the Kaplan–Meier method with the long rank (Mantel–Cox). A P-value of <0.05 was considered as significant.
Survival curves were based on T classification, the actual node classification (N0–N1), and the revised lymph node classification which considered the number of involved nodes (N0, no involved nodes; N1, 1–6 involved nodes; N2, 7–15 involved nodes; N3, 16 or more involved nodes) as in gastric cancer. Moreover, survival was assessed by the Kaplan–Meier method considering N localization for patients with involved nodes in the proximal or distal group.
We also evaluated the survival of patients depending on the number of lymph nodes resected, considering 15 the cut-off number as suggested by Darling [8].
3. Results
Out of 84 patients who underwent surgery for adenocarcinoma of the EGJ we excluded those with R1 and R2 resection (nine and two patients, respectively, 13%) and those who died within the early (30-day) postoperative period (nine patients, 10.7%). Therefore, a total of 64 patients, 39 males (60.9%) and 25 females (39.1%) were included in this study; mean age was 62 years (range: 39–82 years); median follow-up was 29 months (range: 4–72 months). Forty-five patients received adjuvant chemotherapy (67%).
Considering the cardia Siewert classification 46 patients were type I (72%) and 18 patients were type II (28%).
All patients were R0. Above a total of 1216 lymph nodes resected, with a mean of 19 (range: 6–41) retrieved for each patient, 874 (71%) were positive. Forty-two patients (65.6%) had involved lymph nodes; namely 31 (48.5%) in the proximal area, 12 (18.5%) in the distal area too, and none in the distal area only.
Positive lymph nodes were located at: tracheal bifurcation (17 nodes: 2%), lower mediastinum (192 nodes: 22%), paracardial region (341 nodes: 39%), lesser curvature (105 nodes: 12%), bigger curvature (61 nodes: 7%), left gastric artery (70 nodes: 8%), common hepatic artery (44 nodes: 5%), and celiac axis (44 nodes: 5%). Chest lymph nodes were involved in 18 patients (42% of patients with involved lymph nodes), while abdominal lymph nodes were positive in 25 patients (58%). Among Siewert type I patients 15 were N0, while six type II patients had one or more positive lymph nodes.
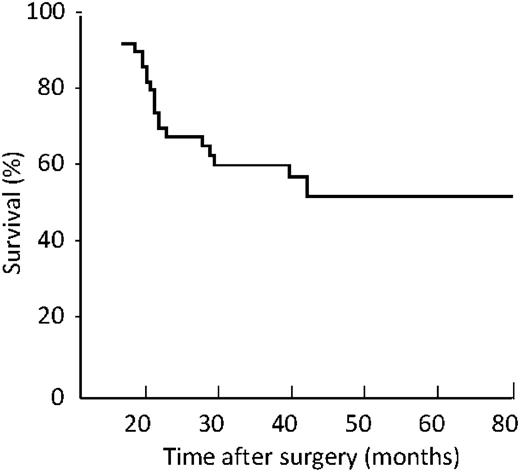
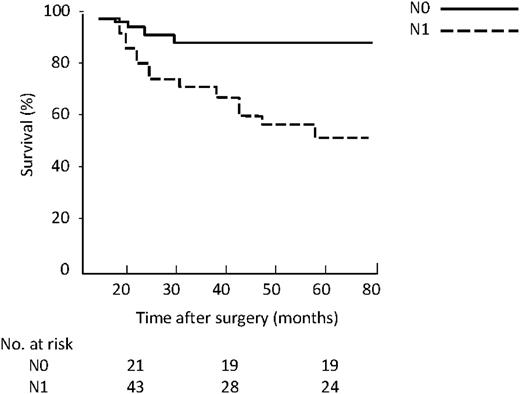
The overall five-year survival was 54% (Fig. 1 ). The Kaplan–Meier survival curves considering the actual T (Fig. 2 ) and N (Fig. 3 ) classification showed a worse prognosis for high T (P<0.023) and N (P=0.031). By this staging system five-year survival was 82% in T1 group, 72% in T2 and 0% in T3 and T4 group, respectively. In N0 and N1 group, five-year survival was 72% and 46%, respectively.
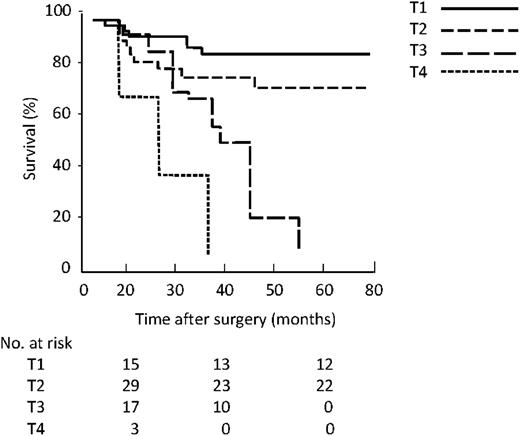
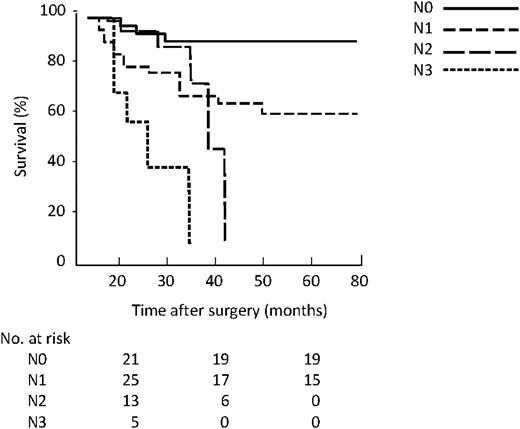
With the revised N classification based on the number of lymph nodes involved, the five-year survival was 91% in the N0 group, 56% in the N1, 0% in N2, and 0% in N3 (Fig. 4 ). There was a significant difference between survival of N0, N1 and N2 (P<0.01) but not between N2 and N3 (P=0.38, log rank Mantel–Cox test).
Kaplan–Meier survival by revised N classification based on number.
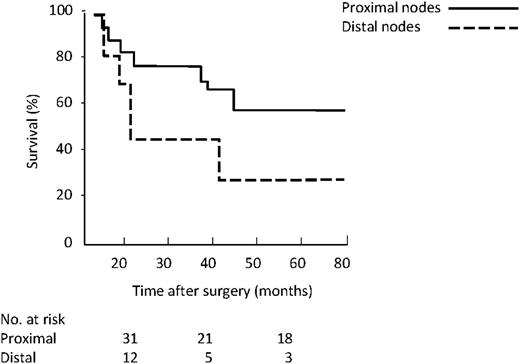
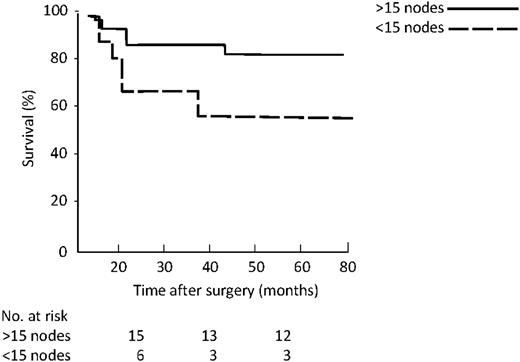
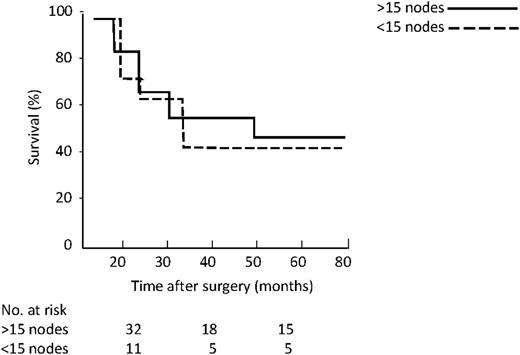
As far as location of involved nodes (Fig. 5 ), the five-year survival was 58% for proximal group vs. 23% for distal (P=0.005). Considering the number of resected lymph nodes, namely more vs. <15, five-year survival in the N0 group (Fig. 6 ) was 83% vs. 57% (P=0.04), while in existing N1 (Fig. 7 ) group it was 46% vs. 41% (P=0.36).
Kaplan–Meier survival by revised N classification based on location.
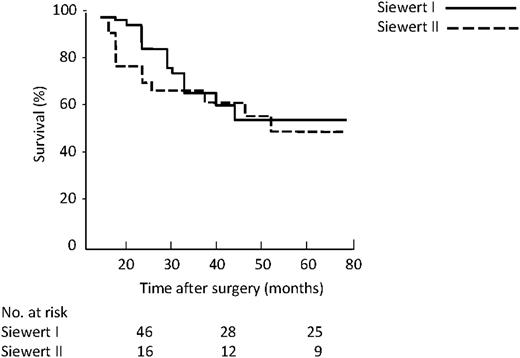
Kaplan–Meier survival related to Siewert classification showed a five-year survival of 54% and 50% for type I and II, respectively (P=0.43) (Fig. 8 ).
4. Discussion
Adenocarcinoma of the EGJ seems to have a different pattern of lymphatic distribution compared to squamous type. The target of surgery for cancer of the EGJ is R0 resection and it is widely accepted that lymph node metastasis is the most important prognostic factor. Yet, notwithstanding the retrospectively reported better outcome after systematic lymphadenectomy, the best possible extent of lymphadenectomy is still a matter of debate [8, 9].
Two-field lymphadenectomy has been proposed to improve staging and outcome even though the prevalence of lymph node metastases in specific stations has not been proven [10]. Most series report 7–40% of mediastinal nodal involvement for type II and III esophagogastric cancer even though abdominal nodes are more affected [11]. In a retrospective study, three-field lymphadenectomy has been reported to provide a more accurate staging although its survival benefit in distal third adenocarcinoma has not been confirmed [12]. Recent studies showed that the number of lymph node metastases for esophageal carcinoma has a better prognostic power compared to the current staging system without distinguishing adenocarcinoma from squamous type [3–7].
After R0 resection the N stage is the strongest prognostic factor for EGJ cancer compared to T as we found in our study [6, 13]. We also showed that a staging considering the number of involved lymph nodes improves the prognostic power compared with the N0–N1 criterion. We observed an important decrease of survival for patients with more than six metastatic nodes, so we suggest that considering three N categories could add more prognostic information by gathering together the groups when more than six lymph nodes are involved.
Recently, Peters et al. [6] proposed a revised N classification based on the number and location of involved nodes in which N1 is considered when one to five nodes on one side of the diaphragm are involved and N2 when six or more are involved, or on both sides of the diaphragm.
The pattern of involved lymph nodes' distribution in our series shows that the most affected region is the paracardial one (39%) followed by the lower mediastinum (22%), lesser curvature of the stomach (12%), left gastric artery (8%), bigger curvature of the stomach (7%), hepatic artery (5%), celiac axis region (5%) and tracheal bifurcation (2%). These lymphatic regions are commonly achieved with the standard lymphadenectomy.
In our series, five-year survival for EGJ type I and II was quite similar. It is true that a larger series is warranted to confirm this result as the number of our patients was too small, however, we underline the fact that the aim of Siewert classification is not prognostic, but for therapeutic decision-making. While almost all authors agree to perform an esophageal resection for type I and a total gastrectomy for type III tumors, the best procedure for type II is still questioned.
Abdominal surgeons often perform gastrectomy for carcinoma of cardia, usually without chest lymphadenectomy. In our series, 24% of patients had one or more positive nodes in the chest. This result suggests the importance of performing a thoracic lymphadenectomy for this tumor even though in our opinion it should not go higher than the carina.
The present study shows also that in patients with adenocarcinoma of the EGJ the prognostic importance of the site of nodes' metastasis is high; the Kaplan–Meier curve clearly shows that the risk of death increased markedly with the distance of nodal metastases from the primary tumor. Moreover, there is an increasing rate of distal nodes involvement as T stage increases. This might suggest a correlation between T stage factor and the presence of distal positive nodes.
In our series, based on distribution of affected lymph nodes, we considered celiac axis, common hepatic artery and tracheal bifurcation as distal regions. The assessment of ≥15 lymph nodes warrants accurate N staging and identification of additional prognostic factors that may enable further refinement of the American Joint Committee for Cancer (AJCC) staging system.
In our series, 92% of patients had 15 or more nodes examined and in N0 patients survival was significantly less if 15 or fewer lymph nodes were examined, thus suggesting an understaging.
Recently, Greenstein et al. suggested the ratio of positive-to-total number of lymph nodes (LNR) as a prognostic criterion since they found that a higher LNR was associated with a worse disease-specific survival and resulted as an independent predictor of prognosis in patients with node-positive esophageal carcinoma [14]. In conclusion, we believe that adenocarcinoma of the EGJ requires a specific lymph node classification that considers the number of nodal metastases, and the site of involved lymph nodes.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
The authors sincerely thank the staff of the Department of General and Thoracic Surgery 2 and Pathology of the Clinico San Carlos Hospital in Madrid for their help in the recording of data.
References
Conference discussion
Dr. T. Dosios (Athens, Greece): In the manuscript you said that you studied 64 patients, but in your presentation I saw 50 patients.
Dr. Ielpo: Yes.
Dr. Dosios: How many are there?
Dr. Ielpo: There are 64, but we didn't have time to record all the data for the Congress, so we recorded data for 50 patients for the Congress. We had a little bit more time to record more patients for the manuscript. So for this reason, there are 64 for the final article.
Dr. Dosios: These patients were suffering from adenocarcinoma of the esophagogastric junction and they underwent a subtotal Ivor–Lewis esophagectomy with proximal gastric resection. You divided the lymph nodes into proximal and distal and you found that involvement of central nodes and removal of fewer than 15 nodes in N0 patients were independent negative predictive factors. Your subsequent conclusion is that the classification of lymph node involvement in adenocarcinoma of the esophagogastric junction following the gastric cancer criteria is adequate for prognostic purposes.
As I understand, the operations were performed in three different low-volume centers, two thoracic and one general surgical, of two different countries within six years.
Dr. Ielpo: No. They were performed in only one department in one hospital in Madrid, but we studied the patients together also with the help of the Tor Vergata University. All of these patients were in one hospital.
Dr. Dosios: The number of surgeons who participated in the study is not reported in the manuscript. Questions are raised whether these surgeons followed the same technique in lymph node sampling throughout the six-year period of this retrospective study. How many surgeons participated in the study and what is the average number of lymph nodes they dissected?
Dr. Ielpo: There were six surgeons, and the department in Madrid is a general surgery department and also thoracic surgery, so they are the same. And the median number of lymph nodes resected for each patient, was almost 19.
Dr. Dosios: No, by each surgeon.
Dr. Ielpo: We don't have the data by each surgeon.
Dr. Dosios: If there is variability in the technique of sampling, this may have a bias on the final results.
Dr. Ielpo: Yes. I don't know exactly the number of lymph nodes resected by each surgeon, but there is not a big variability because the range among the 19 lymph nodes was very little. It was almost 11 lymph nodes to 26.
Dr. Dosios: Finally, the aim of your essay is to compare the current esophageal cancer staging with a new system. You found that 46 out of these 64 patients had lymph node involvement; 36 in the proximal area and 11 in both the distal and proximal areas?
Dr. Ielpo: Yes.
Dr. Dosios: Do you consider these numbers sufficient to draw your strong conclusion regarding the adequacy of the staging system?
Dr. Ielpo: Of course this is not a large series, but this is the experience of one department. What I want to say is that in very few patients it was statistically significant and this fact is very important. I mean with this, the power of the study is stronger. It was a very small series, and, of course, large series are warranted to confirm it.
Dr. G. Decker (Luxembourg): Considering that you found only 2% of patients with positive subcarnial nodes, did you resect the subcarnial nodes in all patients? My second question is, did you study the recurrence and the location of the recurrences?
Dr. Ielpo: In all these patients we performed a two-field lymphadenectomy, and one field is the thoracic one, so we dissected the mediastinal and the subcarinal area too. I also studied the recurrence of these patients, but I don't have the results for this discussion.
Dr. D. Dougenis (Patras, Greece): My question concerns the surgical procedure itself. We do all these gastroesophageal junction tumors through a left thoraco-laparotomy and we can dissect up to the level of the bifurcation of the trachea. Why do you use two incisions instead of one?
Dr. Ielpo: This is the procedure we use in our department for the surgery. I know that you can do it both ways, but this is the experience of my teachers.
Dr. Wood: I think one thing that is always clear is that we have our biases about the way we like to approach the esophagus, and we won't discuss that for this paper.