Abstract
Background
The extracellular matrix metalloproteases MMP-9 and MMP-2 are critical for the invasive potential of tumors. However, it is not clear which of the two plays the predominant role in tumor invasion and progression. In the present study, we compared the clinical efficacy of MMP-9 and MMP-2 overexpression for predicting tumor recurrence and survival after surgical resection in HCC patients.
Materials and Methods
MMP-9 and MMP-2 expression in HCC cell lines and in vitro HCC invasion model were detected by quantitative RT-PCR and immunofluorescence. The expression levels of MMP-9 and MMP-2 were assessed by immunohistochemistry in HCC tissue microarrays from HCC patients (study set) who underwent curative resection. The clinicopathological data were retrospectively analyzed. The results were further verified in an independent cohort of 92 HCC patients (validation set).
Results
Univariate analysis demonstrated that high expression of MMP-9 was associated with both time to recurrence (TTR, P = .015) and overall survival (OS, P = .024), whereas high expression of MMP-2 was only correlated with TTR (P = .041). Multivariate analysis confirmed that MMP-9 expression was an independent predictor of TTR and OS. The coindex of MMP-9 and preoperative serum AFP levels was significantly correlated with TTR and OS (P = .036 and P = .040), but the coindex of MMP-2 and AFP did not show prognostic significance for either TTR or OS (P = .067 and P = .053). The prognostic value of MMP-9 overexpression was validated in the independent data set.
Conclusion
MMP-9 is superior to MMP-2 for the prediction of tumor recurrence and survival in HCC patients after surgical resection.
Similar content being viewed by others
Hepatocellular carcinoma (HCC) is one of the most frequent malignant tumors, and its morbidity and mortality has risen in recent years.1,2 Surgical resection is a main modality for curative therapy and provides a long-term survival of HCC patients. However, the prognosis of HCC patients after surgical resection remains unsatisfactory, with a 5-year recurrence rate as high as 50–70%.3,4 Prediction of recurrence is still a great challenge for the patients who underwent surgical resection. Although some clinicopathological features of HCC, such as tumor multifocality and vascular invasion, are useful to evaluate the prognosis of HCC patients, they cannot meet clinical requirements for precise prediction of HCC course.5 Indeed, there was a marked diversity in outcomes of HCC patients with similar clinicopathological characteristics. Therefore, there were a variety of biomarkers explored to predict outcomes of HCC but still none widely accepted or applied in clinical practice.6
High expression of MMP-2, MMP-9, and both has been associated with tumor progression and poor survival of patients in a variety of cancers, including HCC.7–12 However, there is no consensus on which one plays the predominant role in tumor cell invasion and metastasis, thus limiting their clinical predicative value for the prognosis of HCC patients. Only a few studies have comparatively analyzed MMP-2 and MMP-9 expression simultaneously in HCC, and in these reports, the expression of MMP-9, not MMP-2, was essential for HCC invasion and progression.7,13–15 However, other studies suggested that activation of MMP-2 was also associated with HCC progression.16–19 Our previous work demonstrated that MMP-9, not MMP-2, was significantly upregulated in the formation of 3-dimensional HCC spheroids.20 Thus, MMP-9 appears to possess greater biological activity in tumorigenesis, but whether MMP-9 is more valuable than MMP-2 as a HCC prognostic indicator remains unclear. In the study, we simultaneously detected the expression of MMP-9 and MMP-2 in HCC tissues by immunohistochemistry and evaluated their predictive value in the prognosis of HCC patients after surgical resection.
Materials and Methods
Cell Lines
HCC cells with different metastatic potential (MHCC97-L, MHCC97-H, HCCLM6) were established from same parental cell line at Liver Cancer Institute of Fudan University, and other HCC cells Hep3B with low-metastatic potential were obtained from American Type Culture Collection.
Expression of MMP-9 and MMP-2 in the Formation of an In Vitro HCC Invasion Model
Previously we developed an in vitro 3-D HCC spheroid model by culturing MHCC97-H cells on molecular scaffolds within a rotating wall vessel (RWV) bioreactor.20 Subsequently, we modified this protocol and cocultured highly metastatic MHCC97-H cells and a liver fragment in RWV bioreactor (Synthecon, Houston, TX) to form an in vitro HCC invasion model. Briefly, approximately 1 × 107 suspended MHCC97-H cells in 10 mL of DMEM/F12 medium and a liver fragment (2 × 2 × 2 mm3) from nude mouse were transferred into the RWV bioreactor for long term rotating coculture. After 24-h low-speed rotation culture (7.6 rpm), the speed of RWV bioreactor was further adjusted to maintain cocultures in a freely suspended state. The medium was replaced after 36 h. Spherical cocultures in the RWV bioreactor were collected at different time points of culture (day 0, day 5, day 10, and day 15).
Quantitative RT-PCR
Total RNA was extracted and reverse-transcribed. PCR reaction was performed in a MJ Thermal Cycler and MiniOpticon Real-Time PCR System using the SYBR Green PCR Master Mix kit (Invitrogen, Carlsbad, CA). Gene expression levels were calculated according to: 2−∆CT [∆CT = Ct(target) − Ct(GAPDH)]. The sequences of primers are listed in Table S1.
Cell Immunofluorescence
Cell slides were fixed, blocked, and incubated with primary antibodies against MMP-2 (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA) or MMP-9 (1:100, Cell Signal Technology, Danvers, MA), followed by incubation with FITC-conjugated secondary antibody (1:2000, Invitrogen, Carlsbad, CA). Finally, the slides were counterstained with 4,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA).
Patients and HCC Tissues
A total of 143 HCC patients who underwent curative resection from 1997 to 2000 at the Liver Cancer Institute of Fudan University were included in the study set. Another independent 92 HCC patients with curative resection from 2000 to 2004 were used as validation set. Curative surgical resection was defined as complete excision of the tumors with clear margins and no residue tumor, no tumor thrombus in the portal vein, hepatic veins or the bile duct, and no extrahepatic metastasis. Tumor tissues were collected with informed consent from each patient, and the research protocol was approved by the Ethic Committee of the Zhongshan Hospital. Tumor differentiation grade and tumor staging were determined by the Edmondson–Steiner grading system and the Barcelona Clinic Liver Cancer staging system. Vascular invasion was defined as the tumor involvement of portal vein or hepatic vein. Tumor recurrence was confirmed by dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). The time from the date of surgery to the first detection of tumor recurrence was taken as the time to recurrence (TTR), while the time from surgery to death was defined as overall survival (OS). TTR or OS was censored at last follow-up for the patients without recurrence or still alive.
Tissue Microarray Preparation
Tissue microarrays were prepared as described previously.21 They were derived from two representative histological cores from paraffin-embedded HCC tumor tissue blocks (Shanghai Biochip Co Ltd, Shanghai, China). Pathological analysis of all specimens was reviewed by experienced pathologists.
Immunohistochemistry
Immunohistochemistry was carried out as previously described with little modification.21 Briefly, after antigen retrieval, slides were incubated with primary antibodies against MMP-2 (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA) or MMP-9 (1:100, Cell Signal Technology, Danvers, MA) overnight at 4°C, followed by incubation with biotin-conjugated secondary antibody, and then with horseradish peroxidase-conjugated streptavidin. The sections were stained with DAB and counterstained with hematoxylin. Negative controls were treated identically, but with the primary antibodies omitted. Staining intensity and the percentage of immunoreactive cells were scored by two independent observers blind to the clinical data. Any discrepancies were resolved by consensus. Positive staining of stromal cells in tumor tissues was excluded from the analysis. Staining density was classified by a standard described previously: the negative result of no staining or weak staining was defined as no visible staining or light brown staining in <20% tumor cells; the positive result of moderate or strong staining was defined as brown or dark brown staining in ≥20% tumor cells.18
Statistical Analysis
Statistical calculations were performed using the Statistical Package for the Social Sciences version 11.5 (SPSS, Inc., Chicago, IL). The χ2 test and Fisher exact probability were used for comparison between groups. Cumulative survival time and univariate analysis were calculated by the Kaplan–Meier method and by the log-rank test, respectively. Multivariate analysis employed the Cox multivariate proportional hazard regression model in a stepwise manner (forward, likelihood ratio). The likelihood ratio test was used to compare the predictive efficacy of the biomarkers. A value of P < .05 was considered statistically significant.
Results
Expression of MMP-9 and MMP-2 in HCC Cell Lines and During the Formation of an In Vitro HCC Invasion Model
The expression of MMP-9 was significantly upregulated in the high-metastatic HCC cells HCCLM3 compared with that in low-metastatic HCC cells Hep3B (P < .05), whereas the expression of MMP-2 in HCCLM3 was slightly higher than that in Hep3B (P > .05) (Fig. 1a). In particular, in parallel with metastatic potential increasing of HCC cells (HCCLM3 > MHCC97-H > MHCC97-L > Hep3B), the expression of MMP-9 was upregulated markedly (Fig. 1a). Immunofluorescence also showed that MMP-9 protein was more highly expressed in the cytoplasm of HCCLM3 cells than in Hep3B cells (Fig. 1b), but MMP-2 expression was similar between them (Fig. S1). In addition, an in vitro HCC invasion model was established by 3D coculture of MHCC97-H cells and a liver tissue fragment (Fig. 1c). Compared with MMP-9 level on day 0, MMP-9 expressions in spherical cocultures on day 5, day 10 and day 15 were all significantly upregulated during the formation of HCC invasion model (day 5, P = .001; day 10, P = .047; day 15, P = .003), while MMP-2 expressions on day 10 increased markedly in early stage of invasion (day 10 vs. day 0, P = .012), but the level of MMP-2 on day 15 decreased (Fig. 1d).
Expressions of MMP-2 and MMP-9 in HCC cells and tissues. a The mRNA expression levels of MMP-2 and MMP-9 detected by quantitative RT-PCR in HCC cells with different metastasis potential. *P < .05, compared with low-metastatic HCC cells Hep3B. b Immunofluorescence assay revealed moderate signals of MMP-9 in HCCLM3 cells whereas weak signals in Hep3B (100×). c Microscopic and histological characteristics of the in vitro HCC invasion spheroid. Microscopic morphology of the spheroid with a diameter of 0.8–1.0 cm (a, b, c). Histological analysis with HE stain (50×) showed the processes of adhesion, invasion, and formation of tumor foci (arrow) in the target tissue (Liver, liver fragment). d The mRNA expression of MMP-2 and MMP-9 in the spheroids during the formation of an HCC invasion model. (MMP-2, *compared with day 0, P < .05; MMP-9, **compared with day 0, P < .05). e Representative immunohistochemical staining of MMP-2 (a, b, c) and MMP-9 (d, e, f) in tissue arrays (100×). No and weak stainings were judged as negative (−) results. Moderate and strong stainings were as positive (+) results. a, e no staining; b, f weak staining; c, g moderate staining; d, h strong staining
Clinical Characteristics and Survival Rate of HCC Patients
The clinical characteristics of 143 patients in the study set were showed in Table S2. The median preoperative serum AFP concentration was 607.0 ng/mL (range, 27–6918). The mean tumor size was 6.3 ± 4.3 cm at the time of surgery. The mean follow-up was 61 months (range, 6–133). There were 79 patients (55.2%) who suffered tumor recurrence, and 94 patients (65.7%) died during the time of follow-up. Postoperative cumulative recurrence and survival rates (in brackets) at 3, 5, and 7 years were 41.8% (59.8%), 52.9% (47.5%), and 56.1% (40.1%).
Expression of MMP-2 and MMP-9 in HCC Tissues
Immunohistochemical analysis revealed a variety of staining patterns of MMP-2 and MMP-9. The common patterns were scattered, focal, or diffuse with moderate or strong staining in the cytoplasm of tumor cells. Representative images of tissue stained for MMP-2 and MMP-9 are shown in Fig. 1e. In total, 51.0% of HCC samples (73 of 143) were positive for MMP-2 expression, and 68.5% of HCC (98 of 143) were positive for MMP-9 expression.
Relationship Between the Expression of MMP-2/MMP-9 and Clinicopathological Features
We studied the relationship between the positive expression of MMP-2/MMP-9 and the clinicopathologic variables in HCC patients of study set. As shown in Table 1, the positive expression of MMP-2 was significantly correlated with large tumor size (>5 cm; P = .023), and MMP-9 overexpression was significantly associated with a greater number of tumors (multiple lesion, P = .026).
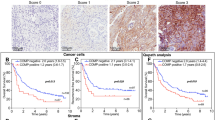
Prognostic Significance of MMP-2 and MMP-9 on HCC Recurrence and Survival
In study set, univariate analysis illustrated that overexpression of MMP-9 was significantly associated with TTR (P = .015) and OS (P = .024) (Fig. 2c, d), while high expression of MMP-2 was only significantly correlated with TTR (P = .041), but not with OS (P = .064) in HCC patients (Fig. 2a, b). Multivariate analysis demonstrated that MMP-9 was an independent predictor for TTR (hazard ratio [HR] = 2.077, 95% confidential interval (95% CI) = 1.212–3.560, P = .008) and OS (HR = 1.858, 95% CI = 1.123–3.074, P = .016) (Table 2). In addition, apart from MMP-9, tumor size, tumor number, and vascular invasion were all associated with OS, and vascular invasion was correlated with TTR in the multivariate analysis (Table 2).
Furthermore, MMP-9 overexpression had prognostic values of TTR in the subgroup of HCC patients with large tumor (>5 cm) (P = .042), single tumor (P = .031) or incomplete tumor encapsulation (P = .035) (Fig. S2). Similarly, MMP-9 could predict OS in the subsets of HCC patients with large tumor (>5 cm) (P = .009), single tumor (P = .030), or incomplete tumor encapsulation (P = .018) (Fig. S2). In contrast, MMP-2 had no significant predictive value for TTR or OS in HCC patients with these characteristics (data not shown).
Prognostic Significance of MMP-2 and MMP-9 on HCC Early and Late Recurrence
Using 36 months postresection as the cutoff time, HCC recurrence was divided into an early recurrence that likely experienced intrahepatic metastasis due to dissemination of the primary tumor cells, and a late recurrence that were more likely to have de novo hepatocarcinogenesis.22 Univariate analysis showed that both MMP-2 and MMP-9 overexpression were significantly associated with HCC early recurrence (P = .011 and P = .013, respectively), but not with late recurrence (P = .267 and P = .801, respectively). Multivariate analysis confirmed that both MMP-2 and MMP-9 had predictive validity for early recurrence (HR = 1.971, 95% CI = 1.138–3.415, P = .016; HR = 2.291, 95% CI = 1.169–4.488, P = .016, respectively), but not for late recurrence (Table 3).
Prognostic Significance of MMP-9 Combined with Serum AFP Level on HCC Recurrence and Survival
As previously reported, serum AFP levels are an unfavorable prognostic factor for HCC patients.23 Univariate analysis indicated that preoperative serum AFP level above 400 ng/mL was significantly associated with shorter TTR (P = .036) and OS (P = .012) (Table 2). Subsequently, we evaluated the prognostic value of MMP-2 or MMP-9 overexpression with serum AFP levels for recurrence and survival of HCC patients. Based on MMP-9 expression and serum AFP values, HCC patients were categorized into three groups with different recurrent risks and prognosis: group I, MMP-9(+) and AFP ≥ 400 ng/mL, high-risk of recurrence and poor prognosis; group II with MMP-9(+) and AFP < 400 ng/mL, or MMP-9(−) and AFP ≥ 400 ng/mL, intermediate-risk of recurrence and intermediate prognosis; group III with MMP-9(−) and AFP < 400 ng/mL, low-risk of recurrence and good prognosis (Fig. 2e, f). Multivariate analyses further demonstrated that the coindex of MMP-9/AFP was an independent prognostic factor for TTR and OS (HR = 2.226, 95% CI = 1.021–4.852, P = .044; HR = 2.057, 95% CI = 1.032–4.102, P = .040), but the coindex of MMP-2/AFP did not have predictive value for either TTR or OS (Table 2).
By logistic regression analysis and the likelihood ratio test, we found that the predictive value of MMP-9 combined with serum AFP level for TTR and OS was better than MMP-9 alone (χ2 = 9.623 > 6.215 for TTR; χ2 = 10.640 > 5.084 for OS, P < .05).
Validation for Prognostic Significance of MMP-9 in the Validation Set
Clinicopathologic features of the cohort of 92 HCC patients were shown in Table S3. MMP-9 overexpression was found to be prognostic for TTR and OS in the validation set (P = .010 and P = .018, Fig. 3c, d), whereas MMP-2 expression was not associated with either TTR (P = .067) or OS (P = .058) (Fig. 3a, b). Multivariate analysis indicated that MMP-9 was an independent prognostic factor for TTR (P = .013) and OS (P = .016), whereas MMP-2 expression was not correlated with TTR and OS in the multivariate analysis (Table S4).
Discussion
The matrix metalloprotease family (MMPs) includes a number of enzymes that participate in extracellular matrix (ECM) remodeling during normal development and tumorigenesis.24 The role of MMPs in tumor growth, metastasis, and angiogenesis has been the focus of intense investigation for many years.25–27 Overexpression of MMPs in tumor cells will enhance degradation of the basement membrane to facilitate invasion of nearby blood vessels (intravasation), followed by extravasion to distant tissues to seed new metastatic sites. Both MMP-9 and MMP-2 are type IV collagenases that show high activity against basement membrane as it is composed primarily of type IV collagen. Thus, these two molecules are critical for tumor cell invasion and metastasis.7–12 However, there was no consensus over which played the predominant role in tumor progression. Our previous work demonstrated that MMP-9, but not MMP-2, was significantly upregulated in HCC spheroids.20 This led us to hypothesize that MMP-9 might be a superior biomarker for HCC prognosis.
By analyzing MMP-2 and MMP-9 expression in a relatively large number of HCC tissue samples, we determined that MMP-9 was superior to MMP-2 for the prediction of HCC recurrence and survival of patients after surgical resection. More importantly, we examined and validated the prognostic effect of MMP-9 overexpression in an independent data set.
We also determined that combination of MMP-9 and serum AFP level was superior to MMP-9 alone for predicting the risk of tumor recurrence and survival of patients. HCC patients can be classified to different subgroups with different risks of tumor recurrence and prognosis according to MMP-9 expression in HCC tissue and preoperative AFP level. Therefore, analysis of MMP-9 expression and serum AFP level may help determine whether adjuvant therapy resection is required after resection.
Previous studies have suggested that MMP-2 and/or MMP-9 are significant biomarkers in HCC patients.9,11,28–30 Here, we establish that MMP-9 expression is superior to MMP-2 expression for predicting HCC recurrence and prognosis after surgical resection. In this study, we found that the expression of MMP-9, but not MMP-2, was consistent with metastatic potentials of HCC cell lines and markedly increased during the formation of an in vitro HCC invasion model. These data provide the evidence that MMP-9 plays more important roles than MMP-2 in the HCC invasiveness and metastasis.
In HCC, MMP-2 was expressed predominantly in tumor stroma cells and only to a lesser extent in carcinoma cells, while MMP-9 was expressed mainly by tumor cells.31 Stromal cells produce regulatory factors that modulate MMP-2 expression of tumor cells.32,33 When tumor cells are separated from stromal cells, as occur in the circulation after vascular intravasation, MMP-2 expression of tumor cells might be downregulated. In contrast, tumor cells may still express MMP-9 and so retain invasive capability for extravasion and metastasis. If this is the case, it would also explain why MMP-9 might be more important than MMP-2 in the process of tumor progression.
Several additional lines of evidence also indicate that MMP-9 is critically important for the invasion and metastasis of HCC.34,35 By comparative analysis of expression profiles without or with intrahepatic metastases, MMP-9 and osteopontin were found to be concordantly upregulated in metastatic HCC.36 Moreover, MMP-9 can directly cleave osteopontin to promote HCC metastasis and can colocalize with F-actin at the front of extending pseudopodia in HCC cells.37,38 As previously reported, increased MMP-9 expression was associated with capsular infiltration of HCC, and levels of plasma MMP-9 were frequently elevated in patients with macroscopic portal vein invasion.15,39 Also, the hepatitis B virus promoted HCC invasion and metastasis by upregulating MMP-9 expression, but had no effect on MMP-2.13,14,30
Although the prognostic value of MMP-9 appears superior to that of MMP-2, MMP-2 is still critical for HCC invasion and metastasis.40,41 Indeed, in our study, we found that MMP-2 expression was associated with HCC early recurrence after surgical resection.
In summary, MMP-9 is superior to MMP-2 as a prognostic biomarker for HCC, and the combination of MMP-9 with serum AFP level is even more efficacious than MMP-9 alone in predicting tumor recurrence and the prognosis of HCC patients.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–6.
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96.
Hao K, Luk JM, Lee NP, Mao M, Zhang C, Ferguson MD, et al. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;9:389.
Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513.
Sakamoto Y, Mafune K, Mori M, Shiraishi T, Imamura H, Mori M, et al. Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. Int J Oncol. 2000;17:237–43.
Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 15;2009:RA32–40.
Zhang Q, Chen X, Zhou J, Zhang L, Zhao Q, Chen G, et al. CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol Ther. 2006;5:808–14.
Bagnoli F, Oliveira VM, Silva MA, Taromaru GC, Rinaldi JF, Aoki T. The interaction between aromatase, metalloproteinase 2,9 and cd44 in breast cancer. Rev Assoc Med Bras. 2010;56:472–7.
Yeh HC, Lin SM, Chen MF, Pan TL, Wang PW, Yeh CT. Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:98–102.
Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, Lupo L, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. 2002;97:425–31.
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, et al. Involvement of PI3 K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009;39:177–86.
Hah N, Lee ST. An absolute role of the PKC-dependent NF-kappaB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;305:428–33.
Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316–22.
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101:387–95.
Kohga K, Tatsumi T, Takehara T, Tsunematsu H, Shimizu S, Yamamoto M, et al. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J Hepatol. 2010;52:872–9.
Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–73.
Torimura T, Ueno T, Kin M, Harada R, Nakamura T, Kawaguchi T, et al. Autocrine motility factor enhances hepatoma cell invasion across the basement membrane through activation of beta1 integrins. Hepatology. 2001;34:62–71.
Tang J, Cui J, Chen R, Guo K, Kang X, Li Y, et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumour Biol. 2011;32:469–79.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9.
Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–21.
Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Johansson N, Ahonen M, Kahari VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000;57:5–15.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67.
Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–20.
Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–66.
Yang P, Yuan W, He J, Wang J, Yu L, Jin X, et al. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: Implications for tumor progression and prognosis. Hepatol Res. 2009;39:1169–77.
Nart D, Yaman B, Yilmaz F, Zeytunlu M, Karasu Z, Kiliç M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010;16:621–30.
Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123–5.
Maatta M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726–34.
Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755–62.
Sawada S, Murakami K, Murata J, Tsukada K, Saiki I. Accumulation of extracellular matrix in the liver induces high metastatic potential of hepatocellular carcinoma to the lung. Int J Oncol. 2001;19:65–70.
Kim JR, Kim CH. Association of a high activity of matrix metalloproteinase-9 to low levels of tissue inhibitors of metalloproteinase-1 and -3 in human hepatitis B-viral hepatoma cells. Int J Biochem Cell Biol. 2004;36:2293–306.
Chung TW, Moon SK, Lee YC, Kim JG, Ko JH, Kim CH. Enhanced expression of matrix metalloproteinase-9 by hepatitis B virus infection in liver cells. Arch Biochem Biophys. 2002;408:147–54.
Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–23.
Takafuji V, Forgues M, Unsworth E, Goldsmith P, Wang XW. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26:6361–71.
Ji XN, Ye SL, Li Y, Tian B, Chen J, Gao DM, et al. Contributions of lung tissue extracts to invasion and migration of human hepatocellular carcinoma cells with various metastatic potentials. J Cancer Res Clin Oncol. 2003;129:556–64.
Hayasaka A, Suzuki N, Fujimoto N, Iwama S, Fukuyama E, Kanda Y, et al. Elevated plasma levels of matrix metalloproteinase-9 (92-kd type IV collagenase/gelatinase B) in hepatocellular carcinoma. Hepatology. 1996;24:1058–62.
Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30:1162–8.
Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009;8:683–8.
Acknowledgment
The authors sincerely thank Professors Jia Fan, Jian Zhou, Shuang-Jian Qiu, and Zhi-Quan Wu for their help in collecting human HCC tissue samples. This study was sponsored by grants from National Natural Science Foundation of China (No. 30772062, No. 81071902, and No. 81000909), China National High-Tech Research and Development Program (2006AA02A-308), China National Key Projects for Infectious Disease (2008ZX10002-021 and 2008ZX 10002-017), Shanghai Pujiang Program (No.08PJ140300), and Shanghai Natural Science Foundation (09ZR1406400).
Author information
Authors and Affiliations
Corresponding author
Additional information
Rongxin Chen and Jiefeng Cui contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, R., Cui, J., Xu, C. et al. The Significance of MMP-9 Over MMP-2 in HCC Invasiveness and Recurrence of Hepatocellular Carcinoma After Curative Resection. Ann Surg Oncol 19 (Suppl 3), 375–384 (2012). https://doi.org/10.1245/s10434-011-1836-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1836-7