Abstract
Background
Pancreatic cancer is one of the most deadly cancers, and serum carbohydrate antigen 19-9 (CA19-9) level has been reported to be a useful prognostic marker in pancreatic cancer. The purpose of this study was to determine which prognostic factor (preoperative or postoperative serum CA19-9 level) is more useful.
Methods
Pre- and postoperative serum CA19-9 levels were measured in 109 patients who underwent surgical resection for pancreatic cancer between 1998 and 2009, and their relationships to clinicopathological factors and overall survival were analyzed with univariate and multivariate methods.
Results
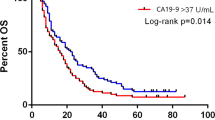
In univariate analysis, tumor location (P = 0.019), postoperative adjuvant chemotherapy (P < 0.001), residual tumor factor status (P < 0.001), UICC pT stage (P = 0.004), lymph node metastasis (P = 0.015), and UICC final stage (P = 0.015) were significantly associated with overall survival. Differences in overall survival were significant between groups divided on the basis of four postoperative CA19-9 cutoff values (37, 100, 200, and 500 U/ml) but not significant between groups divided on the basis of the same four preoperative CA19-9 cutoff values. Pre- to postoperative increase in CA19-9 level also was significantly associated with poor prognosis. In multivariate analysis, postoperative adjuvant chemotherapy (hazard ratio, 1.59; P = 0.004) and postoperative CA19-9 cutoff value of 37 U/ml (HR, 1.64; P = 0.004) remained independent predictors of prognosis.
Conclusions
Postoperative CA19-9 level is a better prognostic factor than preoperative CA19-9 level, and curative surgery for resectable pancreatic cancer should be tried regardless of the preoperative CA19-9 level.

Similar content being viewed by others
References
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210; discussion 210–1.
Shimada K, Sakamoto Y, Sano T, Kosuge T. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery. 2006;139:288–95.
Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43.
Brown KM, Domin C, Aranha GV, Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005;189:278–82.
Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–94.
Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58.
Traverso LW. Pancreatic cancer: surgery alone is not sufficient. Surg Endosc. 2006;20(Suppl 2):S446–9.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Kosuge T, Kiuchi T, Mukai K, Kakizoe T. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol. 2006;36:159–65.
Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–95.
Murakami Y, Uemura K, Sudo T, et al. Impact of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for adenocarcinoma of the body or tail of the pancreas. J Gastrointest Surg. 2009;13:85–92.
Murakami Y, Uemura K, Sudo T, et al. Adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for pancreatic adenocarcinoma. Am J Surg. 2008;195:757–62.
Murakami Y, Uemura K, Sudo T, et al. Postoperative adjuvant chemotherapy improves survival after surgical resection for pancreatic carcinoma. J Gastrointest Surg. 2008;12:534–41.
Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–9.
Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–22.
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-del Castillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–902.
Kang CM, Kim JY, Choi GH, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31–5.
Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008;31:446–53.
Murakami Y, Uemura K, Ohge H, Hayashidani Y, Sudo T, Sueda T. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery. 2006;140:448–53.
Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–5.
Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–3.
(UICC) International Union Against Cancer. TMN classification of malignant tumors. 6th ed. New York: Wiley-Liss; 2002.
Waraya M, Yamashita K, Katagiri H, et al. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol. 2009;16:1231–40.
Fujioka S, Misawa T, Okamoto T, et al. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:539–44.
Ong SL, Garcea G, Thomasset SC, et al. Surrogate markers of resectability in patients undergoing exploration of potentially resectable pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1068–73.
Smith RA, Bosonnet L, Ghaneh P, et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658–66.
Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93:195–9.
Wong D, Ko AH, Hwang J, Venook AP, Bergsland EK, Tempero MA. Serum CA19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas. 2008;37:269–74.
Lundin J, Roberts PJ, Kuusela P, Haglund C. The prognostic value of preoperative serum levels of CA 19-9 and CEA in patients with pancreatic cancer. Br J Cancer. 1994;69:515–9.
Nakao A, Oshima K, Nomoto S, et al. Clinical usefulness of CA-19-9 in pancreatic carcinoma. Semin Surg Oncol. 1998;15:15–22.
Beretta E, Malesci A, Zerbi A, et al. Serum CA 19-9 in the postsurgical follow-up of patients with pancreatic cancer. Cancer. 1987;60:2428–31.
Montgomery RC, Hoffman JP, Riley LB, Rogatko A, Ridge JA, Eisenberg BL. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4:551–6.
Ni XG, Bai XF, Mao YL, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–9.
Zhang S, Wang YM, Sun CD, Lu Y, Wu LQ. Clinical value of serum CA19-9 levels in evaluating resectability of pancreatic carcinoma. World J Gastroenterol. 2008;14:3750–3.
Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2008;15:3512–20.
Schlieman MG, Ho HS, Bold RJ (2003) Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg 138:951–5; discussion 5–6.
Safi F, Schlosser W, Falkenreck S, Beger HG. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253–9.
Yoshimasu T, Maebeya S, Suzuma T, et al. Disappearance curves for tumor markers after resection of intrathoracic malignancies. Int J Biol Markers. 1999;14:99–105.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure of commercial interest: None of the authors have any commercial interest in the subject of this study and received any financial or material support for this study.
Rights and permissions
About this article
Cite this article
Kondo, N., Murakami, Y., Uemura, K. et al. Prognostic Impact of Perioperative Serum CA 19-9 Levels in Patients with Resectable Pancreatic Cancer. Ann Surg Oncol 17, 2321–2329 (2010). https://doi.org/10.1245/s10434-010-1033-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1033-0