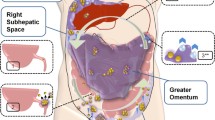
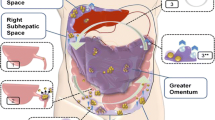
Abstract
Background and Aims
Pseudomyxoma peritonei (PMP) is characterized by peritoneal tumors arising from a perforated appendiceal adenoma or adenocarcinoma, but associated entry of enteric bacteria in the peritoneum has not been considered as a cofactor. Because Gram-negative organisms can upregulate MUC2 mucin gene expression, we determined whether bacteria were detectable in PMP tissues.
Methods
In situ hybridization was performed on resection specimens from five control subjects with noninflamed, nonperforated, non-neoplastic appendix and 16 patients with PMP [six with disseminated peritoneal adenomucinosis (DPAM) and 10 with peritoneal mucinous carcinomatosis (PMCA)]. Specific probes were designed to recognize: (1) 16S rRNA common to multiple bacteria or specific to H. pylori; (2) H. pylori cagA virulence gene; or (3) MUC2 or MUC5AC apomucins. Specimens from one patient with PMCA were examined by ultrastructural immunohistochemistry. Bacterial density and apomucin expression were determined in four histopathological compartments (epithelia, inflammatory cells, stroma, and free mucus).
Results
Enteric bacteria were detected in all specimens. Bacterial density and MUC2 expression were significantly (p < 0.05) higher in PMCA than in DPAM and controls and were highest in free mucin. MUC2 was also expressed in dysplastic epithelia and in associated inflammatory cells. MUC2 expression was significantly correlated with bacterial density.
Conclusions
Multiple enteric bacteria are present in PMP, and bacterial density and MUC2 expression is highest in the malignant form of PMP. Based on these observations, we propose that the bacteria observed in PMP may play a role in the mucinous ascites and perhaps promote carcinogenesis.




Similar content being viewed by others
REFERENCES
Bradley RF, Stewart JH, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551–9
Mann WJ Jr., Wagner J, Chumas J, et al. The management of pseudomyxoma peritonei. Cancer 1990;66:1636–40
Aho AJ, Heinonen R, Lauren P. Benign and malignant mucocele of the appendix. Histological types and prognosis. Acta Chir Scand 1973;139:392–400
Witkamp AJ, de Bree E, Kaag MM, van Slooten GW, van CF, Zoetmulder FA. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg 2001;88:458–63
Galani E, Marx GM, Steer CB, et al. Pseudomyxoma peritonei: the ‘controversial’ disease. Int J Gynecol Cancer 2003;13:413–8
Loungnarath R, Causeret S, Bossard N, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum 2005;48:1372–9
Loungnarath R, Causeret S, Brigand C, et al. [Pseudomyxoma peritonei: new concept and new therapeutic approach]. Ann Chir 2005;130:63–9
Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg 2007;204:943–53
Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994;219:112–9
Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol 2004;11:178–86
Yan H, Pestieau SR, Shmookler BM, et al. Histopathologic analysis in 46 patients with pseudomyxoma peritonei syndrome: failure versus success with a second-look operation. Mod Pathol 2001;14:164–71
Semino-Mora C, Doi SQ, Marty A, et al. Intracellular and interstitial expression of H. pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis 2003;187:1165–77
O’Connell JT, Tomlinson JS, Roberts AA, et al. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol 2002;161:551–64
O’Connell JT, Hacker CM, Barsky SH. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol 2002;15:958–72
Li JD, Dohrman AF, Gallup M, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA 1997;94:967–72
Dohrman A, Miyata S, Gallup M, et al. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1998;1406:251–9
Souei-Mhiri M, Tlili-Graies K, Ben CL, et al. Mucocele of the appendix. Retrospective study of 10 cases. J Radiol 2001;82:463–8
Kuroki Y, Otagiri S, Tsukada K. Disseminated peritoneal adenomucinosis associated with a panperitonitis-like onset: report of a case. Surg Today 2001;31:646–50
Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 1995;19:1390–408
Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun 1993;61:1799–809
Lan Z-D, Dai L, Zhuo X-L, et al. Gene cloning and sequencing of BmK AS and BmK AS-1, two novel neurotoxins from the scorpion Butus martensi Karsch. Toxicon 1999;37:815–23
Bendayan M, Nanci A, Kan FW. Effect of tissue processing on colloidal gold cytochemistry. J Histochem Cytochem 1987;35:983–96
Olivero OA, Chang PK, Lopez-Larraza DM, et al. Preferential formation and decreased removal of cisplatin-DNA adducts in Chinese hamster ovary cell mitochondrial DNA as compared to nuclear DNA. Mutat Res 1997;391:79–86
Semino-Mora C, Dalakas MC. Rimmed vacuoles with beta-amyloid and ubiquitinated filamentous deposits in the muscles of patients with long-standing denervation (postpoliomyelitis muscular atrophy): similarities with inclusion body myositis. Hum Pathol 1998;29:1128–33
Stirling JW, Graff PS. Antigen unmasking for immunoelectron microscopy: labeling is improved by treating with sodium ethoxide or sodium metaperiodate, then heating on retrieval medium. J Histochem Cytochem 1995;43:115–23
Aspholm M, Olfat FO, Norden J, et al. SabA Is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog 2006;2:989–1001
Necchi V, Candusso ME, Tava F, et al. Intracellular, intercellular and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by H. pylori. Gastroenterology 2007;132:1009–23
Oliveira AG, Rocha GA, Rocha AM, et al. Isolation of Helicobacter pylori from the intestinal mucosa of patients with Crohn’s disease. Helicobacter 2006;11:2–9
Thomas JE, Gibson GR, Darboe MK, et al. Isolation of H. pylori from human faeces. Lancet 1992;340:1194–5
Luzzi I, Covacci A, Censini S, et al. Detection of a vacuolating cytotoxin in stools from children with diarrhea. Clin Infect Dis 1996;23:101–6
Kang W, Rathinavelu S, Samuelson LC, et al. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest 2005;85:702–15
Dabbagh K, Takeyama K, Lee HM, et al. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 1999;162:6233–7
Kim YD, Kwon EJ, Park DW, et al. Interleukin-1beta induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol Pharmacol 2002;62:1112–8
Thompson MA, Ashton RW, Pitot HC. Mucinous appendiceal adenocarcinoma presenting 5 years after appendectomy. Ann Intern Med 2004;140:E677–8
Ohara N, Teramoto K. Successful treatment of pseudomyxoma peritonei with intraperitoneal 10 per cent dextrose, sizofiran and cisplatin. J Obstet Gynaecol 2002;22:223
Anonymous. IARC Monograph on the evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. Lyon, France: International Agency for Research on Cancer;1994
Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287:G7–17
Kucharzik T, Maaser C, Lugering A, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis 2006;12:1068–83
Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 2002;160:2253–7
Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 2007;132:551–61
Gatalica Z, Loggie B. COX-2 expression in pseudomyxoma peritonei. Cancer Lett 2006;244:86–90
ACKNOWLEDGMENTS
Supported in part by National Institutes of Health grant CA82312, USUHS grant HU83KT funded by an unrestricted gift in memory of Jessie McAvoy, and the E. Allen Davis Memorial fund. This paper is dedicated to the memory of Jessie McAvoy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the Society of Surgical Oncology, Cancer Symposium. March 23-26, 2006, San Diego, California.
None of the authors have any financial and personal relationship with others that might bias their work, and potential conflicts do not exist.
Rights and permissions
About this article
Cite this article
Semino-Mora, C., Liu, H., McAvoy, T. et al. Pseudomyxoma Peritonei: Is Disease Progression Related to Microbial Agents? A Study of Bacteria, MUC2 and MUC5AC Expression in Disseminated Peritoneal Adenomucinosis and Peritoneal Mucinous Carcinomatosis. Ann Surg Oncol 15, 1414–1423 (2008). https://doi.org/10.1245/s10434-007-9778-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9778-9