-
PDF
- Split View
-
Views
-
Cite
Cite
M. Rizzo, K. Berneis, Low-density lipoprotein size and cardiovascular risk assessment, QJM: An International Journal of Medicine, Volume 99, Issue 1, January 2006, Pages 1–14, https://doi.org/10.1093/qjmed/hci154
Close - Share Icon Share
Abstract
A predominance of small, dense low-density lipoproteins (LDL) has been accepted as an emerging cardiovascular risk factor by the National Cholesterol Education Program Adult Treatment Panel III. LDL size seems to be an important predictor of cardiovascular events and progression of coronary heart disease and evidences suggests that both quality (particularly small, dense LDL) and quantity may increase cardiovascular risk. However, other authors have suggested that LDL size measurement does not add information beyond that obtained by measuring LDL concentration, triglyceride levels and HDL concentrations. Therefore, it remains debatable whether to measure LDL particle size in cardiovascular risk assessment and, if so, in which categories of patient. Therapeutic modulation of LDL particle size or number appears beneficial in reducing the risk of cardiovascular events, but no clear causal relationship has been shown, because of confounding factors, including lipid and non-lipid variables. Studies are needed to investigate the clinical significance of LDL size measurements in patients with coronary and non-coronary forms of atherosclerosis; in particular, to test whether LDL size is associated with even higher vascular risk, and whether LDL size modification may contribute to secondary prevention in such patients.
Introduction
Cardiovascular diseases are still the primary cause of death in most industrialized countries. Effective prevention includes treatment of a series of risk factors: smoking, hypertension, diabetes, obesity, and dyslipidaemia, which includes elevated triglycerides, total and low-density-lipoprotein (LDL) cholesterol levels, as well as lowered high-density-lipoprotein (HDL) cholesterol.1
The therapeutic modulation of distinct LDL subspecies is also of great benefit in reducing the risk of cardiovascular events.1–3 The peak size of LDL in humans shows a bimodal (rather than a normal) distribution, and can be separated into two phenotypes that differ in size, density, physicochemical composition, metabolic behaviour and atherogenicity. These phenotypes have been called ‘pattern A’ (larger, more buoyant LDL) and ‘pattern B’ (smaller, denser LDL predominate).2–5
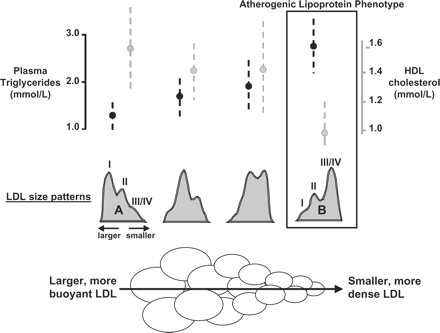
LDL size correlates positively with plasma HDL levels and negatively with plasma triglyceride concentrations, and the combination of small, dense LDL, decreased HDL cholesterol and increased triglycerides has been called the ‘atherogenic lipoprotein phenotype’6 (Figure 1). This partly heritable trait is a feature of the metabolic syndrome, and is associated with increased cardiovascular risk.
LDL heterogeneity and plasma triglyceride and HDL cholesterol concentrations (modified from reference 3).
LDL size seems to be an important predictor of cardiovascular events and progression of coronary artery disease, and a predominance of small, dense LDL has been accepted as an emerging cardiovascular risk factor by the National Cholesterol Education Program Adult Treatment Panel III.1 However, other authors have suggested that LDL subclass measurement does not add independent information to that conferred by the simple LDL concentration, along with the other standard risk factors.7 Thus it remains debatable whether to measure LDL particle size for cardiovascular risk assessment, and if so, in which categories of patients.
LDL size and environmental/genetic influence
The prevalence of the pattern B phenotype is approximately 30% in adult men, 5–10% in young men and women <20 years, and approximately 15–25% in post-menopausal women.2,3 LDL size is genetically influenced, with a heritability ranging from 35–45%, based on an autosomal dominant or codominant model with varying additive and polygenic effects.8 Clearly, non-genetic and environmental factors influence the expression of this phenotype, and abdominal adiposity and oral contraceptive use are both associated with an increase in small, dense LDL.9–11
Dietary factors are also important. A very low-fat high-carbohydrate diet can induce pattern B in people who are genetically predisposed to this phenotype.12 In addition, the predominance of small, dense LDL is commonly found in conjunction with familial disorders of lipoprotein metabolism that are associated with increased risk of premature coronary artery disease, including familial combined hyperlipidaemia, hyper-beta-lipoproteinaemia and hypo-alpha-lipoproteinaemia.2
LDL size measurement
Particle size distribution of plasma LDL subfractions may be measured by different laboratory techniques,7 but the most common procedure is 2–16% gradient gel electrophoresis at 10°C using a Tris (0.09 M)-boric acid (0.08 M)-Na2EDTA (0.003 M) buffer (pH 8.3).9,13 Plasma is adjusted to 20% sucrose, and 3–10 µl are applied to the gel. Potentials are set at 40 mV (15 min), 80 mV (15 min), and 125 mV (24 h). Gels are fixed and stained for lipids in a solution containing oil red O in 60% ethanol at 55°C, and for proteins in a solution containing 0.1% Coomassie brilliant blue R-250, 50% ethanol and 9% acetic acid, and then scanned with a densitometer. Molecular diameters are determined on the basis of migration distance, by comparison with standards of known diameter.9,13
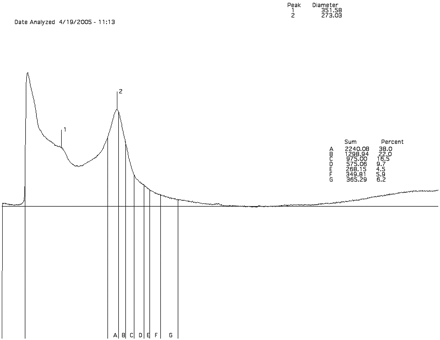
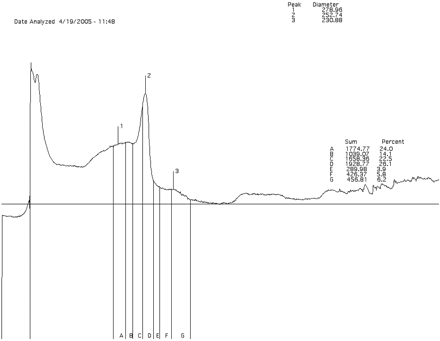
Assignment of LDL subclass phenotypes is based on the particle diameter of the major plasma LDL peak. LDL phenotype A (larger, more buoyant LDL) is defined as an LDL subclass pattern with the major peak at a particle diameter of 258 Å or greater, whereas the major peak of LDL phenotype B (small, dense LDL) is at a particle diameter of <258 Å2,3,9,14 (Figures 2 and 3).
Densitometric scan of lipid-stained 2–16% non-denaturing gradient gel electrophoresis of whole plasma from a subject with a predominance of large, buoyant LDL (LDL phenotype ‘pattern A‘).
Densitometric scan of lipid-stained 2–16% nondenaturing gradient gel electrophoresis of whole plasma from a subject with a predominance of small, dense LDL (LDL phenotype ‘pattern B‘).
Atherogenicity of small, dense LDL
Several reasons have been suggested for the atherogenicity of small, dense LDL. Smaller, denser LDL are more easily taken up by arterial tissue than are larger LDL,15 suggesting greater transendothelial transport of smaller particles. In addition, smaller LDL particles may also have decreased receptor-mediated uptake and increased proteoglycan binding.16 Sialic acid, perhaps because of its exposure at the LDL surface, plays a determinant role in the in vitro association of LDL with the polyanionic proteoglycans,17 and the sialic acid content of LDL particles is lower in subjects with the pattern B phenotype.
Oxidative susceptibility increases and antioxidant concentrations decrease with decreasing LDL size.18 The altered properties of the surface lipid layer associated with a reduced content of free cholesterol19 and increased content of polyunsaturated fatty acids20 might also contribute to the enhanced oxidative susceptibility of small, dense LDL.
Recently,21 we chose apoB transgenic mice to evaluate the kinetic behaviour of human LDL particles of different size in vivo in a genetically homogeneous recipient, thus avoiding other metabolic differences that could influence LDL metabolism. We found that small LDL particles had intrinsic features that led to retarded metabolism and decreased intra/extravascular equilibration compared to medium-sized LDL; these properties could contribute to the greater atherogenicity of small, dense LDL.
Association of LDL size with cardiovascular events and disease progression
The magnitude and independence of the association of LDL size with cardiovascular diseases has been tested in many studies, including cross-sectional and prospective epidemiological studies, as well as clinical intervention trials.22–72 (Table 1). The vast majority (but not all) show a significant univariate association of small, dense LDL with increased coronary heart disease (CHD) risk. However, LDL size is seldom a significant and independent predictor of CHD risk after multivariate adjustment for confounding variables, in particular plasma triglyceride levels and HDL cholesterol concentrations (Table 1).
Univariate and multivariate analyses on the association of size or number of small, dense LDL particles with cardiovascular diseases
| Author . | Study design . | Univariate . | Multivariate . | Author . | Study design . | Univariate . | Multivariate . |
|---|---|---|---|---|---|---|---|
| LDL size | |||||||
| Crouse22 | CS | Y | N | Skoglund47 | CS | Y | Y |
| Austin23 | CS | Y | N | Zambon48 | P | Y | Y |
| Griffin24 | CS | Y | N | Austin49 | P | Y | N |
| Tornvall25 | CS | Y | – | Hulthe50 | CS | N | – |
| Campos26 | CS | Y | N | Hulthe51 | CS | Y | – |
| Coresh27 | CS | Y | N | Bokemark52 | CS | Y | N |
| Griffin28 | CS | Y | Y | Campos53 | P | N | N |
| Campos29 | CS | N | N | Kamigaki54 | CS | Y | N |
| Sherrard30 | CS | N | – | Rosenson55 | P | Y | Y |
| Rajman31 | CS | N | – | Blake56 | P | Y | N |
| Stampfer32 | P | Y | N | Kuller57 | P | Y | N |
| Gardner33 | P | Y | Y | Liu58 | CS | Y | Y |
| Miller34 | P | Y | N | Koba59 | CS | Y | Y |
| Mack35 | P | Y | N | Vakkilainen60 | P | Y | N |
| Lamarche36 | P | Y | Y | Slowik61 | CS | Y | – |
| Gray37 | CS | N | – | Hallman62 | CS | Y | – |
| Wahi38 | CS | N | – | Watanabe63 | CS | Y | – |
| Slyper39 | CS | N | – | Wallenfeldt64 | P | Y | – |
| Freedman40 | CS | Y | N | Kullo65 | CS | Y | N |
| Ruotolo41 | P | Y | N | van Tits66 | P | Y | – |
| O’Neal42 | CS | Y | Y | Inukai67 | CS | Y | Y |
| Landray43 | CS | Y | N | Mohan68 | CS | Y | – |
| Hulthe44 | CS | Y | – | Yoon69 | CS | Y | Y |
| Mykkanen45 | P | N | N | St Pierre70 | P | Y | – |
| Erbey46 | CS | Y | N | Berneis71 | CS | Y | Y |
| LDL number | |||||||
| Rosenson55 | P | Y | Y | Kuller57 | P | Y | Y |
| Blake56 | P | Y | Y | Otvos72 | P | Y | Y |
| Author . | Study design . | Univariate . | Multivariate . | Author . | Study design . | Univariate . | Multivariate . |
|---|---|---|---|---|---|---|---|
| LDL size | |||||||
| Crouse22 | CS | Y | N | Skoglund47 | CS | Y | Y |
| Austin23 | CS | Y | N | Zambon48 | P | Y | Y |
| Griffin24 | CS | Y | N | Austin49 | P | Y | N |
| Tornvall25 | CS | Y | – | Hulthe50 | CS | N | – |
| Campos26 | CS | Y | N | Hulthe51 | CS | Y | – |
| Coresh27 | CS | Y | N | Bokemark52 | CS | Y | N |
| Griffin28 | CS | Y | Y | Campos53 | P | N | N |
| Campos29 | CS | N | N | Kamigaki54 | CS | Y | N |
| Sherrard30 | CS | N | – | Rosenson55 | P | Y | Y |
| Rajman31 | CS | N | – | Blake56 | P | Y | N |
| Stampfer32 | P | Y | N | Kuller57 | P | Y | N |
| Gardner33 | P | Y | Y | Liu58 | CS | Y | Y |
| Miller34 | P | Y | N | Koba59 | CS | Y | Y |
| Mack35 | P | Y | N | Vakkilainen60 | P | Y | N |
| Lamarche36 | P | Y | Y | Slowik61 | CS | Y | – |
| Gray37 | CS | N | – | Hallman62 | CS | Y | – |
| Wahi38 | CS | N | – | Watanabe63 | CS | Y | – |
| Slyper39 | CS | N | – | Wallenfeldt64 | P | Y | – |
| Freedman40 | CS | Y | N | Kullo65 | CS | Y | N |
| Ruotolo41 | P | Y | N | van Tits66 | P | Y | – |
| O’Neal42 | CS | Y | Y | Inukai67 | CS | Y | Y |
| Landray43 | CS | Y | N | Mohan68 | CS | Y | – |
| Hulthe44 | CS | Y | – | Yoon69 | CS | Y | Y |
| Mykkanen45 | P | N | N | St Pierre70 | P | Y | – |
| Erbey46 | CS | Y | N | Berneis71 | CS | Y | Y |
| LDL number | |||||||
| Rosenson55 | P | Y | Y | Kuller57 | P | Y | Y |
| Blake56 | P | Y | Y | Otvos72 | P | Y | Y |
CS, cross-sectional; P, prospective; Y, yes; N, no.
Univariate and multivariate analyses on the association of size or number of small, dense LDL particles with cardiovascular diseases
| Author . | Study design . | Univariate . | Multivariate . | Author . | Study design . | Univariate . | Multivariate . |
|---|---|---|---|---|---|---|---|
| LDL size | |||||||
| Crouse22 | CS | Y | N | Skoglund47 | CS | Y | Y |
| Austin23 | CS | Y | N | Zambon48 | P | Y | Y |
| Griffin24 | CS | Y | N | Austin49 | P | Y | N |
| Tornvall25 | CS | Y | – | Hulthe50 | CS | N | – |
| Campos26 | CS | Y | N | Hulthe51 | CS | Y | – |
| Coresh27 | CS | Y | N | Bokemark52 | CS | Y | N |
| Griffin28 | CS | Y | Y | Campos53 | P | N | N |
| Campos29 | CS | N | N | Kamigaki54 | CS | Y | N |
| Sherrard30 | CS | N | – | Rosenson55 | P | Y | Y |
| Rajman31 | CS | N | – | Blake56 | P | Y | N |
| Stampfer32 | P | Y | N | Kuller57 | P | Y | N |
| Gardner33 | P | Y | Y | Liu58 | CS | Y | Y |
| Miller34 | P | Y | N | Koba59 | CS | Y | Y |
| Mack35 | P | Y | N | Vakkilainen60 | P | Y | N |
| Lamarche36 | P | Y | Y | Slowik61 | CS | Y | – |
| Gray37 | CS | N | – | Hallman62 | CS | Y | – |
| Wahi38 | CS | N | – | Watanabe63 | CS | Y | – |
| Slyper39 | CS | N | – | Wallenfeldt64 | P | Y | – |
| Freedman40 | CS | Y | N | Kullo65 | CS | Y | N |
| Ruotolo41 | P | Y | N | van Tits66 | P | Y | – |
| O’Neal42 | CS | Y | Y | Inukai67 | CS | Y | Y |
| Landray43 | CS | Y | N | Mohan68 | CS | Y | – |
| Hulthe44 | CS | Y | – | Yoon69 | CS | Y | Y |
| Mykkanen45 | P | N | N | St Pierre70 | P | Y | – |
| Erbey46 | CS | Y | N | Berneis71 | CS | Y | Y |
| LDL number | |||||||
| Rosenson55 | P | Y | Y | Kuller57 | P | Y | Y |
| Blake56 | P | Y | Y | Otvos72 | P | Y | Y |
| Author . | Study design . | Univariate . | Multivariate . | Author . | Study design . | Univariate . | Multivariate . |
|---|---|---|---|---|---|---|---|
| LDL size | |||||||
| Crouse22 | CS | Y | N | Skoglund47 | CS | Y | Y |
| Austin23 | CS | Y | N | Zambon48 | P | Y | Y |
| Griffin24 | CS | Y | N | Austin49 | P | Y | N |
| Tornvall25 | CS | Y | – | Hulthe50 | CS | N | – |
| Campos26 | CS | Y | N | Hulthe51 | CS | Y | – |
| Coresh27 | CS | Y | N | Bokemark52 | CS | Y | N |
| Griffin28 | CS | Y | Y | Campos53 | P | N | N |
| Campos29 | CS | N | N | Kamigaki54 | CS | Y | N |
| Sherrard30 | CS | N | – | Rosenson55 | P | Y | Y |
| Rajman31 | CS | N | – | Blake56 | P | Y | N |
| Stampfer32 | P | Y | N | Kuller57 | P | Y | N |
| Gardner33 | P | Y | Y | Liu58 | CS | Y | Y |
| Miller34 | P | Y | N | Koba59 | CS | Y | Y |
| Mack35 | P | Y | N | Vakkilainen60 | P | Y | N |
| Lamarche36 | P | Y | Y | Slowik61 | CS | Y | – |
| Gray37 | CS | N | – | Hallman62 | CS | Y | – |
| Wahi38 | CS | N | – | Watanabe63 | CS | Y | – |
| Slyper39 | CS | N | – | Wallenfeldt64 | P | Y | – |
| Freedman40 | CS | Y | N | Kullo65 | CS | Y | N |
| Ruotolo41 | P | Y | N | van Tits66 | P | Y | – |
| O’Neal42 | CS | Y | Y | Inukai67 | CS | Y | Y |
| Landray43 | CS | Y | N | Mohan68 | CS | Y | – |
| Hulthe44 | CS | Y | – | Yoon69 | CS | Y | Y |
| Mykkanen45 | P | N | N | St Pierre70 | P | Y | – |
| Erbey46 | CS | Y | N | Berneis71 | CS | Y | Y |
| LDL number | |||||||
| Rosenson55 | P | Y | Y | Kuller57 | P | Y | Y |
| Blake56 | P | Y | Y | Otvos72 | P | Y | Y |
CS, cross-sectional; P, prospective; Y, yes; N, no.
Therefore, it may be that the increased risk associated with smaller LDL size in univariate analyses is a consequence of the broader pathophysiology of which small, dense LDL is a part (e.g. high triglycerides, low HDL cholesterol, increased LDL particle number, obesity, insulin resistance, diabetes, metabolic syndrome), rather than a reflection of an intrinsic increased atherogenic potential. A clear causal relationship between small dense LDL and increased cardiovascular risk cannot be proven, based on our present knowledge.
This is further complicated by the fact that at the same level of LDL cholesterol, higher-risk pattern B individuals have significantly more particles than those with pattern A. The number of LDL particles in plasma is potentially important, because the arterial walls are exposed to these particles, and an increased number might increase atherogenicity independently of particle size.73 Is the higher risk of pattern B individuals attributable to the fact that they have more LDL particles in total, or does the smaller size contribute independently to CHD risk?
Assessment of LDL particle size by gradient gel electrophoresis does not provide information about the concentration or number of small, dense LDL particles, which has been historically estimated by measuring apo B concentrations.74 Rader75 and Sniderman76 reviewed 32 trials that studied the relationship between plasma apo B concentrations and CHD risk, but the data did not consistently support a stronger association between CHD risk and apo B then between CHD and other lipid parameters.
LDL particle number is currently assessed by nuclear magnetic resonance, which provides data on both LDL particle size and concentration.77 Higher LDL particle concentrations seem to be important in determining CHD risk,78,79 but few studies have assessed whether the quantity rather than the size of small, dense LDL is more strongly associated with CHD risk.55–57,72 In these studies, the number of total and smaller LDL particles was a significant and independent predictor of CHD risk, after multivariate adjustment for lipid variables55–57,72 (Table 1).
In addition, studies that measured not only LDL cholesterol concentration and particle size, but also LDL particle numbers in plasma, have provided important information on the risk of CHD.73,77 When both number of LDL particles and LDL size are measured in the same study population, small, dense LDL particles are frequently not significantly associated with CHD risk.34,36,41,56,57 (Table 1).
These data underline the clinical importance of assessing LDL particle number in establishing the risk of CHD associated with the presence of small, dense LDL particles.74,77
LDL size and acute myocardial infarction
Acute myocardial infarction and the atherogenic lipoprotein phenotype seem to share a similar array of interrelated metabolic aberrations, including modifications in plasma lipids and lipoproteins as well as a relative resistance to insulin-mediated glucose uptake. The common lipid alterations observed during the acute phase of myocardial infarction include a rise in triglyceride levels and a fall in total, LDL and HDL cholesterol concentrations80–82 and such modifications have a great clinical relevance, since they must be taken into account in making therapeutic decisions.83
However, despite the data regarding modifications of total plasma lipoprotein fractions during a myocardial infarction, it is less certain whether LDL peak particle size is also modified in the acute phase, and therefore what the best time is to measure it. In a group patients admitted to hospital for a myocardial infarction, and followed until discharge and 3 months after the event, reduction of LDL peak particle size was premature and persisted during the hospitalization, with a significant increase 3 months after the myocardial infarction.84 In addition, the timing of these changes seemed to precede those of all other lipoproteins.
Even angina itself (against a background of coronary artery spasm), without atherosclerosis, may lower LDL size.85
LDL size and vascular disease
According to the National Cholesterol Education Program Adult Treatment Panel III, clinical forms of non-coronary atherosclerosis carry a risk for CHD equal to those with established CHD.1 These conditions include peripheral arterial disease, symptomatic (transient ischaemic attack or stroke of carotid origin) and asymptomatic (>50% stenosis on angiography or ultrasound) carotid artery disease and abdominal aortic aneurysm.1
However, despite plentiful data on the relationship between LDL size and atherosclerosis in patients with CHD, very few authors have investigated such relationships in patients with non-coronary forms of atherosclerosis, and studies with larger number of patients are needed. But the available data suggest a strong association between small, dense LDL and non-coronary forms of atherosclerosis.
Smaller, denser LDL particles are a risk factor for peripheral arterial disease, whether in the absence or presence of diabetes.42 In some studies, common features of peripheral arterial disease are represented by increased triglyceride levels and lower HDL cholesterol concentrations,42 and patients with such lipid abnormalities mostly have atherogenic small, dense LDL particles.6,86
The association between carotid disease and small, dense LDL has been found in healthy subjects43,47,51,52,62 as well as in various categories of patients (familial combined hyperlipidemia, familial hypercholesterolemia, vascular dementia, Alzheimer's disease, insulin resistance or type 2 diabetes).44,58,63,66,71 It has also been recently suggested that carotid atherosclerosis regression or progression may be linked to LDL size.64,66
No published studies have directly examined the association of LDL size with the presence of abdominal aortic aneurysms. However, patients with abdominal aortic aneurysms show an elevated prevalence of the metabolic syndrome,87 which is associated with the predominance of small, dense LDL,2,3,6 and therefore such patients are likely to have reduced LDL size.
Clinical value of therapeutic modification of LDL size
Hypolipidaemic treatment can alter LDL subclass distribution, and statins and fibrates are currently the most widely used lipid-lowering agents. Statins are potent inhibitors of hydroxy-methyl-glutaryl-coenzyme A reductase, the rate-limiting enzyme in hepatic cholesterol synthesis, and are the primary drugs of choice for the treatment of elevated plasma LDL cholesterol concentrations.74 Fibrates have a major impact on triglyceride metabolism, mediated by peroxisomal proliferation activator receptors (PPAR) and through stimulation of lipoprotein lipase.88
Statins potentially lower large, medium and small LDL particles, but with wide variation between the different agents. Treatment with pravastatin favourably altered LDL size in four studies,89–92 but not in others.93–104 Similarly, simvastatin therapy showed significant,105–110 moderate111,112 or no effect113–124 on LDL subclasses. Fluvastatin and atorvastatin seem to be more effective: fluvastatin favourably altered LDL size in six studies,125–130 but not in two; 131,132 treatment with atorvastatin was more often beneficial104,109,133–148 than not.124,149–158 Promising data were also recently published on the use of rosuvastatin.159
Fibrates seem to have more effect than statins on LDL size. Therapy with fenofibrate, bezafibrate and gemfibrozil usually results in a beneficial effect,100,101,107,109,117,118,145,149,151,160–176 with very rare negative findings.113,177
As already reported,88 although not directly demonstrated, modulation of LDL size with fibrates probably contributed to the reduction of CHD risk in the Helsinki Heart Study and in the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trials Study Group.178–180 It is also likely that there was a bias towards small LDL in clinical trials that showed cardiovascular benefit from statins.181–183
Other studies have investigated whether therapeutic modification of LDL particle size and number reduces cardiovascular risk, using arteriographic changes as outcome variables. CAD progression in the controls is significantly greater in patients with a predominance of small, dense LDL,35,184 and arteriographic benefit is concentrated in patients with a predominance of small, dense LDL who receive treatment that tends to lower it. These studies included the Stanford Coronary Risk Intervention Project, the Familial Atherosclerosis Treatment Study (FATS), the St Thomas’ Atherosclerosis Regression Study (STARS) and the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial.34,48,55,185
In all of these studies, therapeutic modulation of LDL size was significantly associated with reduced CHD risk on univariate analysis. Under multivariate analysis with adjustments for confounding factors, changes in LDL size by drug therapy were the best correlates of changes in coronary stenosis in FATS,48 and the smallest LDL fraction was the plasma lipoprotein subfraction with the single most powerful effect on CAD regression in middle-aged men with hypercholesterolaemia in STARS.185
In PLAC-I, using a logistic regression models that adjusted for lipid levels and other confounding factors, elevated levels of small LDL were associated with a nine-fold increased risk of CAD progression, but only in the placebo group.55 In addition, in this study, elevated LDL particle number was a predictor of CAD progression after adjustment for race, sex, age, treatment group, baseline lumen diameter and plasma lipids.55
All these data suggest that the therapeutic modification of LDL size, or number of small LDL particles, is significantly associated with reduced cardiovascular risk, even after multivariate adjustment for confounding factors. However, whether LDL size or number of LDL particles is more (or equally) important can not be concluded from the current evidence.
Conclusions
Genetic and environmental factors influence the expression of small, dense LDL, which is not completely independent of traditional lipids, correlating negatively with plasma HDL concentrations and positively with plasma triglyceride levels. Small, dense LDL are associated with the metabolic syndrome, and with increased risk for cardiovascular disease and diabetes mellitus.
LDL size also seems to be an important predictor of cardiovascular events, and progression of coronary artery disease and a predominance of small, dense LDL has been accepted as an emerging cardiovascular risk factor by the National Cholesterol Education Program Adult Treatment Panel III.1 However, other authors have suggested that LDL subclass measurement does not add independent information to that conferred generically by the LDL concentration along with the other standard risk factors.8 The number, rather than the density, of LDL particles may be a stronger predictor of CHD.71
Therefore, remains debatable whether to measure LDL particle size in cardiovascular risk assessment, and if so, in which categories of patients. In several studies, therapeutical modulation of LDL particle size or number has been of great benefit in reducing the risk of cardiovascular events, but a no clear causal relationship has been shown, due to confounding factors, including lipid and non-lipid variables. Additional studies are needed to investigate the clinical significance of LDL size measurement.
Recently, the Coordinating Committee of the National Cholesterol Education Program stated that very high-risk patients may benefit from more aggressive therapeutic approaches.186 Screening for the presence of small, dense LDL in patients with coronary or non-coronary forms of atherosclerosis may identify those with even higher vascular risk, and assist in the targeting of appropriate treatment.
References
National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report.
Rizzo M, Berneis K. Should we measure routinely the LDL peak particle size?
Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism.
Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity.
Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk.
Sacks FM, Campos H. Low-Density Lipoprotein Size and Cardiovascular Disease: A Reappraisal.
Austin MA. Genetic epidemiology of low-density lipoprotein subclass phenotypes.
Rizzo M, Barbagallo CM, Severino M, Polizzi F, Onorato F, Noto D, Cefalu AB, Pace A, Marino G, Notarbartolo A, Averna RM: Low-density-lipoprotein peak particle size in a Mediterranean population.
Terry RB, Wood PD, Haskell WL, Stefanick ML, Krauss RM. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men.
de Graaf J, Swinkels DW, Demacker PN, de Haan AF, Stalenhoef AF. Differences in the low density lipoprotein subfraction profile between oral contraceptive users and controls.
Dreon DM, Fernstrom HA, Williams PT, Krauss RM. LDL subclass patterns and lipoprotein response to a low-fat, high-carbohydrate diet in women.
Rizzo M, Taylor JM, Barbagallo CM, Berneis KK, Blanche PJ, Krauss RM. Effects on lipoprotein subclasses of combined expression of human hepatic lipase and human apoB in transgenic rabbits.
Georgieva AM, van Greevenbroek MMJ, Krauss RM, Brouwers MCGJ, Vermeulen VM M-J, Robertus-Teunissen MG, van der Kallen CJH, de Bruin TWA. Subclasses of low-density lipoprotein and very low-density lipoprotein in familial combined hyperlipidemia: relationship to multiple lipoprotein phenotype.
Bjornheden T, Babyi A, Bondjers G, Wiklund O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system.
Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity.
Camejo G, Lopez A, Lopez F, Quinones J. Interaction of low density lipoproteins with arterial proteoglycans. The role of charge and sialic acid content.
Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins.
Tribble DL, Holl LG, Wood PD, Krauss RM. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size.
de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects.
Berneis K, Shames DM, Blanche PJ, La Belle M, Rizzo M, Krauss RM. Plasma clearance of human low-density lipoprotein in human apolipoprotein B transgenic mice is related to particle diameter.
Crouse JR, Parks JS, Schey HM, Kahl FR. Studies of low density lipoprotein molecular weight in human beings with coronary artery disease.
Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction.
Griffin BA, Caslake MJ, Yip B, Tait GW, Packard CJ, Shepherd J. Rapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugation.
Tornvall P, Karpe F, Carlson LA, Hamsten A. Relationships of low density lipoprotein subfractions to angiographically defined coronary disease in young survivors of myocardial infarction.
Campos H, Genest J, Blijlevens E, McNamara JR, Jenner JL, Ordovas JM, Wilson PW, Schaefer EJ. Low density lipoprotein particle size and coronary artery disease.
Coresh J, Kwiterovich PO Jr, Smith HH, Bachorik PS. Association of plasma triglyceride concentration and LDL particle diameter, density, and chemical composition with premature coronary artery disease in men and women.
Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small dense LDL to coronary heart disease risk.
Campos H, Roederer GO, Lussier-Cacan S, Davignon J, Krauss RM. Predominance of large LDL and reduced HDL2 cholesterol in normolipidemic with coronary artery disease.
Sherrard B, Simpson H, Cameron J, Wahi S, Jennings G, Dart A. LDL particle size in subjects with previously unsuspected coronary heart disease: relationship with other cardiovascular risk markers.
Rajman I, Kendall MJ, Cramb R, Holder RL, Salih M, Gammage MD. Investigation of low density lipoprotein subfractions as a coronary risk factor in normotriglyceridaemic men.
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction.
Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women.
Miller BD, Alderman EL, Haskell WL, Fair JM, Krauss RM. Predominance of dense low-density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project.
Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS). Treatment effects and relation to coronary angiographic progression.
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. P results from the Quebec Cardiovascular Study.
Gray RS, Robbins DC, Wang W, Yeh JL, Fabsitz RR, Cowan LD, Welty TK, Lee ET, Krauss RM, Howard BV. Relation of LDL size to the insulin resistance syndrome and coronary heart disease in American Indians. The Strong Heart Study.
Wahi S, Gatzka CD, Sherrard B, Simpson H, Collins V, Dowse G, Zimmet P, Jennings G, Dart AM. Risk factors for coronary heart disease in a population with a high prevalence of obesity and diabetes: a case-control study of the Polynesian population of Western Samoa.
Slyper AH, Zvereva S, Schectman G, Hoffmann RG, Walker JA. Lowdensity lipoprotein particle size is not a discriminating marker for atherogenic risk in male offspring of parents with early coronary artery disease.
Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease.
Ruotolo G, Ericsson CG, Tettamanti C, Karpe F, Grip L, Svane B, Nilsson J, de Faire U, Hamsten A. Treatment effects on serum lipoprotein lipids, apolipoproteins and low density lipoprotein particle size and relationships of lipoprotein variables to progression of coronary artery disease in the Bezafibrate Coronary Atherosclerosis Intervention Trial (BECAIT).
O’Neal DN, Lewicki J, Ansari MZ, et al. Lipid levels and peripheral vascular disease in diabetic and non-diabetic subjects.
Landray MJ, Sagar G, Muskin J, Murray S, Holder RL, Lip GYH. Association of atherogenic low-density lipoprotein subfractions with carotid atherosclerosis.
Hulthe J, Wiklund O, Olsson G, Fagerberg B, Bokemark L, Nivall S, Wikstrand J. Computerized measurement of LDL particle size in human serum. Reproducibility studies and evaluation of LDL particle size in relation to metabolic variables and the occurrence of atherosclerosis.
Mykkanen L, Kuusisto J, Haffner S, Laakso M, AustinMA. LDL size and risk of coronary heart disease in elderly men and women.
Erbey JR, Robbins D, Forrest KY, Orchard TJ. Low-density lipoprotein particle size and coronary artery disease in a childhood-onset type 1 diabetes population.
Skoglund-Andersson C, Tang R, Bond MG, de Faire U, Hamsten A, Karpe F. LDL particle size distribution is associated with carotid intima-media thickness in healthy 50-year-old men.
Zambon A, Hokanson JE, Brown BG, Brunzell JD. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density.
Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, Sharp DS. Low-density lipoprotein particle size, triglycerides, and highdensity lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men.
Hulthe J, Wiklund O, Bondjers G, Wikstrand J. LDL particle size in relation to intima-media thickness and plaque occurrence in the carotid and femoral arteries in patients with hypercholesterolaemia.
Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study.
Bokemark L, Wikstrand J, Attvall S, Hulthe J, Wedel H, Fagerberg B. Insulin resistance and intima-media thickness in the carotid and femoral arteries of clinically healthy 58-year-old men. The Atherosclerosis and Insulin Resistance Study (AIR).
Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low-density lipoprotein size, pravastatin treatment, and coronary events.
Kamigaki AS, Siscovick DS, Schwartz SM, Psaty BM, Edwards KL, Raghunathan TE, Austin MA. Low density lipoprotein particle size and risk of early-onset myocardial infarction in women.
Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial.
Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women.
Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study.
Liu ML, Ylitalo K, Nuotio I, Salonen R, Salonen JT, Taskinen MR. Association between carotid intima-media thickness and low-density lipoprotein size and susceptibility of low-density lipoprotein to oxidation in asymptomatic members of familial combined hyperlipidemia families.
Koba S, Hirano T, Kondo T, Shibata M, Suzuki H, Murakami M, Geshi E, Katagiri T. Significance of small dense low-density lipoproteins and other risk factors in patients with various types of coronary heart disease.
Vakkilainen J, Pajukanta P, Cantor RM, Nuotio IO, Lahdenpera S, Ylitalo K, Pihlajamaki J, Kovanen PT, Laakso M, Viikari JS, Peltonen L, Taskinen MR. Genetic influences contributing to LDL particle size in familial combined hyperlipidaemia.
Slowik A, Iskra T, Turaj W, Hartwich J, Dembinska-Kiec A, Szczudlik A. LDL phenotype B and other lipid abnormalities in patients with large vessel disease and small vessel disease.
Hallman DM, Brown SA, Ballantyne CM, Sharrett AR, Boerwinkle E. Relationship between low-density lipoprotein subclasses and asymptomatic atherosclerosis in subjects from the Atherosclerosis Risk in Communities (ARIC) Study.
Watanabe T, Koba S, Kawamura M, Itokawa M, Idei T, Nakagawa Y, Iguchi T, Katagiri T. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia.
Wallenfeldt K, Bokemark L, Wikstrand J, Hulthe J, Fagerberg B. Apolipoprotein B/apolipoprotein A-I in relation to the metabolic syndrome and change in carotid artery intima-media thickness during 3 years in middle-aged men.
Kullo IJ, Bailey KR, McConnell JP, Peyser PA, Bielak LF, Kardia SL, Sheedy PF 2nd, Boerwinkle E, Turner ST. Low-density lipoprotein particle size and coronary atherosclerosis in subjects belonging to hypertensive sibships.
van Tits LJ, Smilde TJ, van Wissen S, de Graaf J, Kastelein JJ, Stalenhoef AF. Effects of atorvastatin and simvastatin on low-density lipoprotein subfraction profile, low-density lipoprotein oxidizability, and antibodies to oxidized low-density lipoprotein in relation to carotid intima media thickness in familial hypercholesterolemia.
Inukai T, Yamamoto R, Suetsugu M, Matsumoto S, Wakabayashi S, Inukai Y, Matsutomo R, Takebayashi K, Aso Y. Small low-density lipoprotein and small low-density lipoprotein/total low-density lipoprotein are closely associated with intima-media thickness of the carotid artery in Type 2 diabetic patients.
Mohan V, Deepa R, Velmurugan K, Gokulakrishnan K. Association of small dense LDL with coronary artery disease and diabetes in urban Asian Indians – the Chennai Urban Rural Epidemiology Study (CURES-8).
Yoon Y, Song J, Park HD, Park KU, Kim JQ. Significance of small dense low-density lipoproteins as coronary risk factor in diabetic and non-diabetic Korean populations.
St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study.
Berneis K, Jeanneret C, Muser J, Felix B, Miserez AR. Low-density lipoprotein size and subclasses are markers of clinically apparent and non-apparent atherosclerosis in type 2 diabetes.
Otvos JD, Freedman DS, Pegus C.
Lada AT, Rudel LL. Associations of low density lipoprotein particle composition with atherogenicity.
Lamarche B, Lemieux I, Despres JP. The small, dense LDL phenotype and the risk of coronary heart disease: epidemiology, patho-physiology and therapeutic aspects.
Rader DJ, Hoeg JM, Brewer HB Jr. Quantitation of plasma apolipoproteins in the primary and secondary prevention of coronary artery disease.
Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, Walldius G. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment.
Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease.
Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance.
Otvos J, Cronwell W, Shalaurova I. LDL particle but not LDL cholesterol are highly elevated in the metabolic syndrome. Results from the Framingham Offspring Study.
Avogaro P, Bon GB, Cazzolato G, et al. Variations in apolipoproteins B and A1 during the course of myocardial infarction.
Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism.
Carlsson R, Lindberg G, Westin L, Israelsson B. Serum lipids four weeks after acute myocardial infarction are a valid basis for lipid lowering intervention in patients receiving thrombolysis.
Kingswood JC, Williams S, Owens DR. How soon after myocardial infarction should plasma lipid values be assessed?
Barbagallo CM, Rizzo M, Cefalù AB, et al. Changes in plasma lipids and low-density lipoprotein peak particle size during and after myocardial infarction.
Miwa K. Low density lipoprotein particles are small in patients with coronary vasospasm.
Rizzo M, Berneis K. Lipid triad of atherogenic lipoprotein phenotype: a role in cardiovascular prevention?
Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL; SMART Study Group. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm.
Marais AD. Therapeutic modulation of low-density lipoprotein size.
O’Keefe JH Jr, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia.
Otvos JD, Shalaurova I, Freedman DS, Rosenson RS. Effects of pravastatin treatment on lipoprotein subclass profiles and particle size in the PLAC-I trial.
Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial.
Masana L, Villoria J, Sust M, Ros E, Plana N, Perez-Jimenez F, Franco M, Olivan JJ, Pinto X, Videla S. Treatment of type IIb familial combined hyperlipidemia with the combination pravastatin-piperazine sultosilate.
Vega GL, Krauss RM, Grundy SM. Pravastatin therapy in primary moderate hypercholesterolaemia: changes in metabolism of apolipoprotein B-containing lipoproteins.
Cheung MC, Austin MA, Moulin P, Wolf AC, Cryer D, Knopp RH. Effects of pravastatin on apolipoprotein-specific high density lipoprotein subpopulations and low density lipoprotein subclass phenotypes in patients with primary hypercholesterolemia.
Contacos C, Barter PJ, Sullivan DR. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia.
Zambon S, Cortella A, Sartore G, Baldo-Enzi G, Manzato E, Crepaldi G. Pravastatin treatment in combined hyperlipidaemia. Effect on plasma lipoprotein levels and size.
Franceschini G, Cassinotti M, Vecchio G, Gianfranceschi G, Pazzucconi F, Murakami T, Sirtori M, D’Acquarica AL, Sirtori CR. Pravastatin effectively lowers LDL cholesterol in familial combined hyperlipidemia without changing LDL subclass pattern.
Guerin M, Dolphin PJ, Talussot C, Gardette J, Berthezene F, Chapman MJ. Pravastatin modulates cholesteryl ester transfer from HDL to apoB-containing lipoproteins and lipoprotein subspecies profile in familial hypercholesterolemia.
Contacos C, Barter PJ, Vrga L, Sullivan DR. Cholesteryl ester transfer in hypercholesterolaemia: fasting and postprandial studies with and without pravastatin.
Rustemeijer C, Schouten JA, Voerman HJ, Hensgens HE, Donker AJ, Heine RJ. Pravastatin compared to bezafibrate in the treatment of dyslipidemia in insulin-treated patients with type 2 diabetes mellitus.
Lemieux I, Laperriere L, Dzavik V, Tremblay G, Bourgeois J, Despres JP. A 16-week fenofibrate treatment increases LDL particle size in type IIA dyslipidemic patients.
Blake GJ, Albert MA, Rifai N, Ridker PM. Effect of pravastatin on LDL particle concentration as determined by NMR spectroscopy: a substudy of a randomized placebo controlled trial.
Kazama H, Usui S, Okazaki M, Hosoi T, Ito H, Orimo H. Effects of bezafibrate and pravastatin on remnant-like lipoprotein particles and lipoprotein subclasses in type 2 diabetes.
Sirtori CR, Calabresi L, Pisciotta L, Cattin L, Pauciullo P, Montagnani M, Manzato E, Bittolo Bon G, Fellin R. Effect of statins on LDL particle size in patients with familial combined hyperlipidemia: a comparison between atorvastatin and pravastatin.
Homma Y, Ozawa H, Kobayashi T, Yamaguchi H, Sakane H, Nakamura H. Effects of simvastatin on plasma lipoprotein subfractions, cholesterol esterification rate, and cholesteryl ester transfer protein in type II hyperlipoproteinemia.
Kontopoulos AG, Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Mayroudi MC, Boudoulas H. Effects of simvastatin and ciprofibrate alone and in combination on lipid profile, plasma fibrinogen and low density lipoprotein particle structure and distribution in patients with familial combined hyperlipidaemia and coronary artery disease.
Nestel P, Simons L, Barter P, Clifton P, Colquhoun D, Hamilton-Craig I, Sikaris K, Sullivan D. A comparative study of the efficacy of simvastatin and gemfibrozil in combined hyperlipoproteinemia: prediction of response by baseline lipids, apo E genotype, lipoprotein(a) and insulin.
Forster LF, Stewart G, Bedford D, Stewart JP, Rogers E, Shepherd J, Packard CJ, Caslake MJ. Influence of atorvastatin and simvastatin on apolipoprotein B metabolism in moderate combined hyperlipidemic subjects with low VLDL and LDL fractional clearance rates.
Vega GL, Ma PT, Cater NB, Filipchuk N, Meguro S, Garcia-Garcia AB, Grundy SM. Effects of adding fenofibrate (200 mg/day) to simvastatin (10 mg/day) in patients with combined hyperlipidemia and metabolic syndrome.
Bays HE, McGovern ME. Once-daily niacin extended release/lovastatin combination tablet has more favorable effects on lipoprotein particle size and subclass distribution than atorvastatin and simvastatin.
Wakatsuki A, Ikenoue N, Izumiya C, Okatani Y, Sagara Y. Effect of estrogen and simvastatin on low-density lipoprotein subclasses in hypercholesterolemic postmenopausal women.
Wakatsuki A, Okatani Y, Ikenoue N. Effects of combination therapy with estrogen plus simvastatin on lipoprotein metabolism in postmenopausal women with type IIa hypercholesterolemia.
Nakandakare E, Garcia RC, Rocha JC, Sperotto G, Oliveira HC, Quintao EC. Effects of simvastatin, bezafibrate and gemfibrozil on the quantity and composition of plasma lipoproteins.
Zhao SP, Hollaar L, van‘t Hooft FM, Smelt AH, Gevers Leuven JA, van der Laarse A. Effect of simvastatin on the apparent size of LDL particles in patients with type IIB hyperlipoproteinemia.
Gaw A, Packard CJ, Murray EF, Lindsay GM, Griffin BA, Caslake MJ, Vallance BD, Lorimer AR, Shepherd J. Effects of simvastatin on apoB metabolism and LDL subfraction distribution.
de Graaf J, Demacker PN, Stalenhoef AF. The effect of simvastatin treatment on the low-density lipoprotein subfraction profile and composition in familial hypercholesterolaemia.
Bredie SJ, de Bruin TW, Demacker PN, Kastelein JJ, Stalenhoef AF. Comparison of gemfibrozil versus simvastatin in familial combined hyperlipidemia and effects on apolipoprotein-B-containing lipoproteins, low-density lipoprotein subfraction profile, and low-density lipoprotein oxidizability.
Jeck T, Riesen WF, Keller U. Comparison of bezafibrate and simvastatin in the treatment of dyslipidaemia in patients with NIDDM.
Hoogerbrugge N, Jansen H, De Heide L, Zillikens MC, Deckers JW, Birkenhager JC. The additional effects of acipimox to simvastatin in the treatment of combined hyperlipidaemia.
Lagrost L, Athias A, Lemort N, Richard JL, Desrumaux C, Chatenet-Duchene L, Courtois M, Farnier M, Jacotot B, Braschi S, Gambert P. Plasma lipoprotein distribution and lipid transfer activities in patients with type IIb hyperlipidemia treated with simvastatin.
Nishikawa O, Mune M, Miyano M, Nishide T, Nishide I, Maeda A, Kimura K, Takahashi T, Kishino M, Tone Y, Otani H, Ogawa A, Maeda T, Yukawa S. Effect of simvastatin on the lipid profile of hemodialysis patients.
Geiss HC, Schwandt P, Parhofer KG. Influence of simvastatin on LDL-subtypes in patients with heterozygous familial hypercholesterolemia and in patients with diabetes mellitus and mixed hyperlipoproteinemia.
van den Akker JM, Bredie SJ, Diepenveen SH, van Tits LJ, Stalenhoef AF, van Leusen R. Atorvastatin and simvastatin in patients on hemodialysis: effects on lipoproteins, C-reactive protein and in vivo oxidized LDL.
van Tits LJ, Smilde TJ, van Wissen S, de Graaf J, Kastelein JJ, Stalenhoef AF. Effects of atorvastatin and simvastatin on low-density lipoprotein subfraction profile, low-density lipoprotein oxidizability, and antibodies to oxidized low-density lipoprotein in relation to carotid intima media thickness in familial hypercholesterolemia.
Moriguchi EH, Vieira JL, Itakura H. Differences in the effects of fluvastatin on lipoprotein subclasses distribution is dependent on triglyceride levels.
Marz W, Scharnagl H, Abletshauser C, Hoffmann MM, Berg A, Keul J, Wieland H, Baumstark MW. Fluvastatin lowers atherogenic dense low-density lipoproteins in postmenopausal women with the atherogenic lipoprotein phenotype.
Winkler K, Abletshauser C, Hoffmann MM, Friedrich I, Baumstark MW, Wieland H, Marz W. Effect of fluvastatin slow-release on low density lipoprotein (LDL) subfractions in patients with type 2 diabetes mellitus: baseline LDL profile determines specific mode of action.
Yoshino G, Hirano T, Kazumi T, Takemoto M, Ohashi N. Fluvastatin increases LDL particle size and reduces oxidative stress in patients with hyperlipidemia.
Winkler K, Abletshauser C, Friedrich I, Hoffmann MM, Wieland H, Marz W. Fluvastatin slow-release lowers platelet-activating factor acetyl hydrolase activity: a placebo-controlled trial in patients with type 2 diabetes.
Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N. Fluvastatin improves endothelial dysfunction in overweight postmenopausal women through small dense low-density lipoprotein reduction.
Yuan JN, Tsai MY, Hegland J, Hunninghake DB. Effects of fluvastatin (XU 62–320), an HMG-CoA reductase inhibitor, on the distribution and composition of low density lipoprotein subspecies in humans.
Superko HR, Krauss RM, DiRicco C. Effect of fluvastatin on low-density lipoprotein peak particle diameter.
Hoogerbrugge N, Jansen H. Atorvastatin increases low-density lipoprotein size and enhances high-density lipoprotein cholesterol concentration in male, but not in female patients with familial hypercholesterolemia.
Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles.
Nordoy A, Hansen JB, Brox J, Svensson B. Effects of atorvastatin and omega-3 fatty acids on LDL subfractions and postprandial hyperlipemia in patients with combined hyperlipemia.
Freed MI, Ratner R, Marcovina SM, Kreider MM, Biswas N, Cohen BR, Brunzell JD; Rosiglitazone Study 108 investigators. Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus.
Pontrelli L, Parris W, Adeli K, Cheung RC. Atorvastatin treatment beneficially alters the lipoprotein profile and increases low-density lipoprotein particle diameter in patients with combined dyslipidemia and impaired fasting glucose/type 2 diabetes.
Forster LF, Stewart G, Bedford D, Stewart JP, Rogers E, Shepherd J, Packard CJ, Caslake MJ. Influence of atorvastatin and simvastatin on apolipoprotein B metabolism in moderate combined hyperlipidemic subjects with low VLDL and LDL fractional clearance rates.
Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles.
Sasaki S, Kuwahara N, Kunitomo K, Harada S, Yamada T, Azuma A, Takeda K, Nakagawa M. Effects of atorvastatin on oxidized low-density lipoprotein, low-density lipoprotein subfraction distribution, and remnant lipoprotein in patients with mixed hyperlipoproteinemia.
Tsimihodimos V, Karabina SA, Tambaki AP, Bairaktari E, Goudevenos JA, Chapman MJ, Elisaf M, Tselepis AD. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB.
Sakabe K, Fukuda N, Wakayama K, Nada T, Shinohara H, Tamura Y. Effects of atorvastatin therapy on the low-density lipoprotein subfraction, remnant-like particles cholesterol, and oxidized low-density lipoprotein within 2 weeks in hypercholesterolemic patients.
Lariviere M, Lamarche B, Pirro M, Hogue JC, Bergeron J, Gagne C, Couture P. Effects of atorvastatin on electrophoretic characteristics of LDL particles among subjects with heterozygous familial hypercholesterolemia.
Lemieux I, Salomon H, Despres JP. Contribution of apo CIII reduction to the greater effect of 12-week micronized fenofibrate than atorvastatin therapy on triglyceride levels and LDL size in dyslipidemic patients.
Wagner AM, Jorba O, Bonet R, Ordonez-Llanos J, Perez A. Efficacy of atorvastatin and gemfibrozil, alone and in low dose combination, in the treatment of diabetic dyslipidemia.
Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW, Mancuso JP, Rader DJ. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol.
Lins RL, Matthys KE, Billiouw JM, Dratwa M, Dupont P, Lameire NH, Peeters PC, Stolear JC, Tielemans C, Maes B, Verpooten GA, Ducobu J, Carpentier YA. Lipid and apoprotein changes during atorvastatin up-titration in hemodialysis patients with hypercholesterolemia: a placebo-controlled study.
O’Keefe JH Jr, Captain BK, Jones PG, Harris WS. Atorvastatin reduces remnant lipoproteins and small, dense low-density lipoproteins regardless of the baseline lipid pattern.
Frost RJ, Otto C, Geiss HC, Schwandt P, Parhofer KG. Effects of atorvastatin versus fenofibrate on lipoprotein profiles, low-density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia.
Geiss HC, Otto C, Schwandt P, Parhofer KG. Effect of atorvastatin on low-density lipoprotein subtypes in patients with different forms of hyperlipoproteinemia and control subjects.
Melenovsky V, Malik J, Wichterle D, Simek J, Pisarikova A, Skrha J, Poledne R, Stavek P, Ceska R. Comparison of the effects of atorvastatin or fenofibrate on nonlipid biochemical risk factors and the LDL particle size in subjects with combined hyperlipidemia.
Tsimihodimos V, Karabina SA, Tambaki A, Bairaktari E, Achimastos A, Tselepis A, Elisaf M. Effect of atorvastatin on the concentration, relative distribution, and chemical composition of lipoprotein subfractions in patients with dyslipidemias of type IIA and IIB.
Soedamah-Muthu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, Fuller JH, Julier K, Mackness MI, Neil HA; CARDS Investigators. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease.
Manuel-Y-Keenoy B, Van Campenhout C, Vertommen J, De Leeuw I. Effects of Atorvastatin on LDL sub-fractions and peroxidation in type 1 diabetic patients: a randomised double-blind placebo-controlled study.
van den Akker JM, Bredie SJ, Diepenveen SH, van Tits LJ, Stalenhoef AF, van Leusen R. Atorvastatin and simvastatin in patients on hemodialysis: effects on lipoproteins, C-reactive protein and in vivo oxidized LDL.
Empen K, Geiss HC, Lehrke M, Otto C, Schwandt P, Parhofer KG. Effect of atorvastatin on lipid parameters, LDL subtype distribution, hemorrheological parameters and adhesion molecule concentrations in patients with hypertriglyceridemia.
Ikejiri A, Hirano T, Murayama S, Yoshino G, Gushiken N, Hyodo T, Taira T, Adachi M. Effects of atorvastatin on triglyceride-rich lipoproteins, low-density lipoprotein subclass, and C-reactive protein in hemodialysis patients.
Dornbrook-Lavender KA, Joy MS, Denu-Ciocca CJ, Chin H, Hogan SL, Pieper JA. Effects of atorvastatin on low-density lipoprotein cholesterol phenotype and C-reactive protein levels in patients undergoing long-term dialysis.
Caslake MJ, Stewart G, Day SP, Daly E, McTaggart F, Chapman MJ, Durrington P, Laggner P, Mackness M, Pears J, Packard CJ. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia.
Packard CJ. LDL subfractions and atherogenicity: an hypothesis from the University of Glasgow.
Vakkilainen J, Mero N, Schweizer A, Foley JE, Taskinen MR. Effects of nateglinide and glibenclamide on postprandial lipid and glucose metabolism in type 2 diabetes.
Badiou S, Merle De Boever C, Dupuy AM, Baillat V, Cristol JP, Reynes J. Fenofibrate improves the atherogenic lipid profile and enhances LDL resistance to oxidation in HIV-positive adults.
Tsimihodimos V, Kakafika A, Tambaki AP, Bairaktari E, Chapman MJ, Elisaf M, Tselepis AD. Fenofibrate induces HDL-associated PAF-AH but attenuates enzyme activity associated with apoB-containing lipoproteins.
Guerin M, Bruckert E, Dolphin PJ, Turpin G, Chapman MJ. Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia.
Chapman MJ, Guerin M, Bruckert E. Atherogenic, dense low-density lipoproteins. Pathophysiology and new therapeutic approaches.
Feher MD, Caslake M, Foxton J, Cox A, Packard CJ. Atherogenic lipoprotein phenotype in type 2 diabetes: reversal with micronised fenofibrate.
Tan CE, Chew LS, Tai ES, Chio LF, Lim HS, Loh LM, Shepherd J. Benefits of micronised Fenofibrate in type 2 diabetes mellitus subjects with good glycemic control.
Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Comparative effects of cerivastatin and fenofibrate on the atherogenic lipoprotein phenotype in proteinuric renal disease.
Yuan J, Tsai MY, Hunninghake DB. Changes in composition and distribution of LDL subspecies in hypertriglyceridemic and hypercholesterolemic patients during gemfibrozil therapy.
Yoshida H, Ishikawa T, Ayaori M, Shige H, Ito T, Suzukawa M, Nakamura H. Beneficial effect of gemfibrozil on the chemical composition and oxidative susceptibility of low density lipoprotein: a randomized, double-blind, placebo-controlled study.
Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, Hamsten A, Taskinen MR; DAIS Group. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS).
Farnier M, Freeman MW, Macdonell G, Perevozskaya I, Davies MJ, Mitchel YB, Gumbiner B; the Ezetimibe Study Group. Efficacy and safety of the coadministration of ezetimibe with fenofibrate in patients with mixed hyperlipidaemia.
Ikewaki K, Tohyama J, Nakata Y, Wakikawa T, Kido T, Mochizuki S. Fenofibrate effectively reduces remnants, and small dense LDL, and increases HDL particle number in hypertriglyceridemic men – a nuclear magnetic resonance study.
Ikewaki K, Noma K, Tohyama J, Kido T, Mochizuki S. Effects of bezafibrate on lipoprotein subclasses and inflammatory markers in patients with hypertriglyceridemia–a nuclear magnetic resonance study.
Badiou S, De Boever CM, Dupuy AM, Baillat V, Cristol JP, Reynes J. Small dense LDL and atherogenic lipid profile in HIV-positive adults: influence of lopinavir/ritonavir-containing regimen.
Superko RH, Berneis KK, William PT, Rizzo M, Wood PD. Gemfibrozil reduces small low-density lipoprotein more in normolipemic subjects classified as low-density lipoprotein pattern B compared with pattern A.
Hokanson JE, Austin MA, Zambon A, Brunzell JD. Plasma triglyceride and LDL heterogeneity in familial combined hyperlipidemia.
Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study.
Tenkanen L, Manttari M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and the treatment with gemfibrozil. Experience from the Helsinki Heart Study.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol: Veterans Affairs High-density Lipoprotein Cholesterol Intervention Trials Study Group.
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels.
The Long-term Intervention with Pravastatin in Ischemic Heart Disease (LIPID) Study Group. Prevention of cardiovascular events and deaths with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels.
Downs JR, Beere PA, Whitney E, et al. Design and rationale of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).
Williams PT, Superko HR, Haskell WL, Alderman EA, Blanche PJ, Holl LG, Krauss RM. Smallest LDL particles are most strongly related to coronary disease progression in men.
Watts GF, Mandalia S, Brunt JHN, et al. Independent association between plasma lipoprotein subfraction levels and the course of coronary artery disease in the St Thomas’ Atherosclerosis regression study (STARS).
Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, et al. for the Coordinating Committee of the National Cholesterol Education Program. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.
Author notes
From the 1Department of Clinical Medicine and Emerging Diseases, University of Palermo, Italy, and 2Department of Endocrinology and Diabetology, University Hospital Zurich, Switzerland