Abstract
The -1082A>G polymorphism is located in promoter region of interleukin-10 (IL-10) and it could affect the production of IL-10. Numerous studies have investigated the association between IL-10 -1082A>G and risk of digestive cancer. However, the conclusion is still inconsistent. Here, we have performed a meta-analysis and systematic review to determine the association between the IL-10 -1082A>G and susceptibility to digestive cancer. In this meta-analysis, we identified 40 eligible studies, involving 7195 patients of digestive cancer and 11755 controls. By pooling all eligible studies, we found the variant -1082G allele significantly increased risk of digestive cancer (G vs. A: OR = 1.181, 95% CI: 1.057–1.319). Further stratified analysis was performed to evaluate the influence of cancer types, ethnicities, study design, sample size and Hardy–Weinberg equilibrium. Stratified analysis suggested that, the -1082A>G polymorphism was only associated with increased risk for gastric cancer (G vs. A: OR = 1.281, 95% CI: 1.102–1.488) and in Asian population (G vs. A: OR = 1.399, 95% CI: 1.188–1.646). No significant publication bias was detected. Based on 40 studies and 18950 participants, we found the variant IL-10 -1082G allele significantly increased susceptibility to digestive cancer, especially for gastric cancer and in Asian population.
Similar content being viewed by others
Introduction
Cytokines have been investigated for decades and many important cytokines are involved in human diseases, such as interleukin-1 and osteoarthritis1,2. In 1989, Mosmann and colleagues3 first reported a cytokine named “cytokine synthesis inhibiting factor (CISF)”, which was secreted by T helper 2 (Th2) clones and inhibited synthesis of interferon-γ (IFN-γ) in Th1 clones. The CISF is now known as interleukin-10 (IL-10).
IL-10 is a cytokine with potent anti-inflammatory activity4, produced by macropahges, T helper 2 cells and B lymphocytes5,6. IL-10 is a multifunctional cytokine involved in both innate and adaptive immune response4,6. As an inflammatory cytokine, IL-10 participates in the development of various diseases, such as kidney disease7, heart failure8, chronic infection9,10 and cancer10. Although IL-10 has been extensively studied, the exact role of IL-10 in cancer is still elusive, since evidence suggested that IL-10 could mediate both anticancer immune response and immune-mediated rejection of cancer6.
The gene encoding IL-10 is located on chromosome 1 (1q31-1q32). Three single nucleotide polymorphisms (SNPs) have been confirmed in the promoter region of IL-10: -592C>A (rs1800872), -819C>T (rs1800871) and -1082A>G (rs1800896). Previous studies have shown that the three polymorphisms could affect the expression of IL-1011,12 and alter the susceptibility to digestive cancers13,14,15. In addition to the elusive role of IL-10 in cancer development, the relationship between functional polymorphisms in IL-10 promoter region and cancer risk is also mysterious. Several meta-analyses have been performed to evaluate the association between IL-10 polymorphisms and cancer risk16,17; however, the association between -1082A>G polymorphism and digestive cancer has not been assessed. Thus, this meta-analysis was performed to investigate the association between 1082 polymorphism and digestive cancer and assess the influence of confounding factors.
Methods
Searching strategy
This meta-analysis were conducted and reported in corresponding to the PRISMA guidelines of systematic reviews and meta-analyses (see Supplementary Table S1 online)18. Online databases of PubMed, EMBASE and CNKI were searched. The following terms were used: “Interleukin-10” or “IL10”, “polymorphisms, single nucleotide” or “SNPs” and “cancer” or “ neoplasm”. Both plain text and medical subheadings of above key words were used for searching. No limitation of origin, languages, or other items was placed. To identify additional studies, references of previous meta-analyses and reviews were also manually searched.
Inclusion criteria
Records identified from databases were first screened by titles and abstracts and then full-text articles were further reviewed. Eligible studies were judged by the following criteria: (1) case-control studies; (2) investigating the association between IL-10 -1082A>G polymorphism and digestive cancer risk; (3) available genotype distribution data. According to the inclusion criteria, 2 authors (LC and TW) extracted eligible studies independently. The two authors reached consensus on each records.
Data extraction
Name of first author, year of publication, country where the study was carried out, cancer type, ethnicity, the source of control, number of cases and controls, genotype frequency in cases and controls were collected from eligible studies. Ethnicity was simply classified as Asian, Caucasian and Latino (Table 1). Included studies were defined as hospital-based (HB) and population-based (PB) according to the source of control. Sample size of eligible studies was classified as large (>500) or small (<500). All data were extracted by two authors (LC and TW) independently with a predesigned data-collection form. Two authors reached consensus on each item.
Quality assessment
“Methodological quality assessment scale” (the scale can be found as Supplementary Table S2 online), a quality scale modified form previous meta-analyses19, was used to evaluate methodological quality of eligible studies. Briefly, the following items were assessed: the representativeness of cases, source of controls, ascertainment of relevant cancer, sample size, quality control of genotyping methods and Hardy-Weinberg equilibrium (HWE). Quality scores ranged from 0 to 10 (0: the lowest; 10: the highest).
Statistical analysis
Odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated to estimate the association strength between IL-10 -1082A>G polymorphism and digestive cancer risk. Deviation from the Hardy–Weinberg equilibrium (HWE) among controls subjects was tested by a x2-test and a P<0.05 was considered as significant disequilibrium. The pooled ORs were calculated for allele comparison (G vs. A), homozygote comparison (GG vs. AA), heterozygote comparison (GA vs. AA) and dominant models (GG/GA vs. AA, considering the dominant effect of the IL-10 -1082G allele). For some studies only combined genotype (GG/G) data was reported20,21, thus, only dominant comparison models were conducted for these studies. Heterogeneity between studies were determined by chi-square based on Q test and the random-effects model was used when there was significant heterogeneity (P<0.1); otherwise, the fixed-effects model was applied22. Sub-group analyses were conducted according to cancer types, ethnicities, source of control, HWE and sample size. Sub-group analysis was not performed for those subgroups with less than 2 studies. When significant heterogeneity presented, meta-regression was performed to detect the source of heterogeneity. Egger's test and Begg's test were used to test publication bias and a p < 0.05 was significant23. Sensitivity analysis was performed to assess individual studies' effect on the pooled results. All meta-analyses were calculated by STATA (version 10.0; Stata Corp, College Station, Texas USA). And all P values are two-side.
Results
Overview of eligible studies
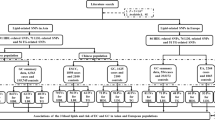
According to our searching strategy, 752 records were retrieved and screened. After primary screening, 38 full-text papers were retrieved for further assessment13,14,15,20,21,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56. The study reported by Zhou SZ et al was excluded for lacking of detail genotype distribution data56. In the studies reported by El-Omar EM29, Guo W32 and Savage SA14, both gastric cancer and esophageal cancer were reported and the data were presented independently and each kind of the cancer was treated as a separate study. Thus, 40 eligible studies were included in this meta-analysis13,14,15,20,21,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. The process of study selection was shown in Figure 1.
Of the 40 eligible studies, 7195 patients of digestive cancer and 11755 controls were enrolled. Baseline characteristics of those studies were shown in Table 1. Most studies were performed among Asian population (24 studies) and Caucasian population (11 studies).
Methodological quality of eligible studies was assessed by a quality scale reported by previous studies. Generally, quality of eligible studies was acceptable, with an average score of 7.3. Of 40 analyzed studies, 23 were hospital-based and 17 studies were population based. As for HWE, 24 studies were in agreement with HWE, 13 studies were in disagreement with HWE and it was unable to test in 3 studies20,21,44 due to combined data (Table 2). Since no genotyping error was reported, all studies were included in quantitative synthesis and stratified analysis was performed to assess the influence of disagreement of HWE.
Meta-analysis Results
By pooling all eligible studies, compared with the wild -1082A allele, we found the variant IL-10 -1082G allele was associated with significantly increased risk of digestive cancer in all four comparison models (G vs. A: OR = 1.181, 95% CI: 1.057–1.319; Heterogeneity, P<0.001; Figure 2 and Figure 3; Table 2).
Further sub-group analyses were conducted to assess the effects of potential confounding factors. When stratified by cancer types, we found the variant G allele only increased risk of gastric cancer (G vs. A: OR = 1.281, 95% CI: 1.102–1.488; Heterogeneity, P<0.001) but did not alter the risk of colorectal cancer (G vs. A: OR = 0.937, 95% CI: 0.805–1.090; Heterogeneity, P = 0.710), hepatocellular carcinoma (G vs. A: OR = 1.104, 95% CI: 0.797–1.530; Heterogeneity, P = 0.283) or esophageal cancer (G vs. A: OR = 0.982, 95% CI: 0.820–1.175; Heterogeneity, P = 0.591). As for ethnicities, significant association was only found among Asians (G vs. A: OR = 1.399, 95% CI: 1.188–1.646; Heterogeneity, P<0.001), while the -1082 polymorphism did not alter digestive cancer risk in Caucasians (G vs. A: OR = 1.016, 95% CI: 0.930–1.111; Heterogeneity, P = 0.796). HWE also significantly affected the pooled analysis. In the sub-groups classified according to source of control and sample size, meta-analysis results were quite consistent.
Heterogeneity and publication bias
Notably, significant heterogeneity was observed in most comparisons. Thus, meta-regression was performed to detect the source of heterogeneity. The results indicated that sample size (P<0.001), HWE (P<0.001), source of control (P<0.001) and cancer types (P = 0.03) contributed heterogeneity, while ethnicity (P = 0.207) did not. Publication bias was detected by Egger's test and Begg's test and no evidence of publication bias was found (P = 0.133 for Begg's test and P = 0.524 for Egger's test; Figure 4). As shown in Figures 2,3,4, the study reported by Alpizar-Alpizar W24 was an outlier. Thus, sensitivity analysis was performed to assess individual study's effect by omitting one study each time. Sensitivity analysis revealed that the pooled results were not affected by the study by Alpizar-Alpizar W et al or any other studies (see supplementary Figure S3 online), indicating our results were stable and reliable.
Discussion
In this meta-analysis, we identified 40 eligible studies, including 7195 cases and 11755 controls. By pooling all eligible studies, we found the variant IL-10 -1082G allele significantly increased the susceptibility to digestive cancer, especially to gastric cancer and among Asian population.
By pooling all eligible studies, we found the IL-10 -1082A>G polymorphism was associated with significantly increased risk of digestive cancer in all comparison models. Then stratified analysis showed that the increased risk was mostly contributed by gastric cancer, since significant association was observed only in gastric cancer and ORs in the sub-groups of gastric cancer were similar with those in overall analysis. It has been proposed that inflammation is a risk factor of tumorigenesis57. In the process of chronic gastric inflammation, different types of cytokines are secreted by activated neutrophils and mononuclear cells and altered cytokine levels have been observed36. Thus, it is biological plausible that IL-10 polymorphism increased risk of gastric cancer. Sub-group analysis revealed that the IL-10 -1082A>G polymorphism was only associated with gastric cancer and no association was found for other digestive cancers, indicating that the role of IL-10 varied among cancers.
During sub-group analysis for ethnicities, we found ethnicity significantly affect the association between IL-10 -1082A>G polymorphism and digestive cancer risk. Since the variant -1082G allele was only associated with increased risk in Asian population and no significant association was found in Caucasian population. This ethnicity difference is common for meta-analysis and may be explained by different environmental exposure, life style and genetic background. Of note, since the incidence of gastric cancer was higher in Asian population, most Asian studies were about gastric cancer (19 of 24 studies, as shown in table 1). The higher prevalence of gastric cancer in Asian population might be another explanation for the ethnicity difference.
In the process of statistical analysis, we found the study reported by Alpizar-Alpizar W24 and colleagues was an outlier. This could be explained by ethnicity difference, since the study was conducted among Latinos. Additionally, the frequency of IL-10 -1082G allele was very low in Alpizar-Alpizar's study24. Specifically, the GG and GA genotype was not detected in cases while the GG was not detected in controls and only one participants carried the heterozygote GA genotype in controls (0% in cases and 1.14% in controls), which would led to relatively wide confidence intervals as shown in Figure 2 and Figure 3. It should also be highlighted that this study was conducted in high-risk population, which might be also related with the low frequency of G allele.
In this meta-analysis, we included 40 studies with 18950 participants. The sample size was large enough to provide enough statistical power. Additionally, no publication bias was detected by Egger's test and Begg's test, suggesting our results were unbiased. On the other hand, limitation of this meta-analysis should also be also highlighted. Firstly, heterogeneity was significant in this meta-analysis. Due to the significant heterogeneity, we used random-effects model to calculate the pooled ORs, which could provide stable results. To identify the source of heterogeneity, meta-regression was conducted and revealed that sample size, HWE, source of control and cancer types were the sources. And stratified analyses were also performed to evaluate the influence of these confounding factors. Secondly, individual data were missed and we could not assess the effects of other factors, like environmental factors, life habit and family history.
In summary, in this meta-analysis of 40 studies and 18950 participants, we found the variant IL-10 -1082G allele significantly increased susceptibility to digestive cancer, especially for gastric cancer and in Asian population.
References
Lee, A. S. et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527, 440–447 (2013).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7, 33–42 (2011).
Fiorentino, D. F., Bond, M. W. & Mosmann, T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170, 2081–2095 (1989).
Mosser, D. M. & Zhang, X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226, 205–218 (2008).
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19, 683–765 (2001).
Mocellin, S., Marincola, F. M. & Young, H. A. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 78, 1043–1051 (2005).
Sinuani, I., Beberashvili, I., Averbukh, Z. & Sandbank, J. Role of IL-10 in the progression of kidney disease. World J Transplant 3, 91–98 (2013).
Kaur, K. et al. Biology of TNFalpha and IL-10 and their imbalance in heart failure. Heart Fail Rev 14, 113–123 (2009).
O'Garra, A., Barrat, F. J., Castro, A. G., Vicari, A. & Hawrylowicz, C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev 223, 114–131 (2008).
Asadullah, K., Sterry, W. & Volk, H. D. Interleukin-10 therapy--review of a new approach. Pharmacol Rev 55, 241–269 (2003).
Suarez, A., Castro, P., Alonso, R., Mozo, L. & Gutierrez, C. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation 75, 711–717 (2003).
Turner, D. M. et al. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 24, 1–8 (1997).
Bouzgarrou, N. et al. Combined analysis of interferon-gamma and interleukin-10 gene polymorphisms and chronic hepatitis C severity. Hum Immunol 70, 230–236 (2009).
Savage, S. A. et al. Polymorphisms in interleukin -2, -6 and -10 are not associated with gastric cardia or esophageal cancer in a high-risk chinese population. Cancer Epidemiol Biomarkers Prev 13, 1547–1549 (2004).
Liu, J. et al. Polymorphisms of interleukin-10 promoter are not associated with prognosis of advanced gastric cancer. World J Gastroenterol 17, 1362–1367 (2011).
Yu, Z., Liu, Q., Huang, C., Wu, M. & Li, G. The interleukin 10 -819C/T polymorphism and cancer risk: a HuGE review and meta-analysis of 73 studies including 15,942 cases and 22,336 controls. OMICS 17, 200–214 (2013).
Xue, H. et al. A meta-analysis of interleukin-10 -592 promoter polymorphism associated with gastric cancer risk. PLoS One 7, e39868 (2012).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6, e1000100 (2009).
Qiu, M. T. et al. Hsa-miR-499 rs3746444 polymorphism contributes to cancer risk: a meta-analysis of 12 studies. PLoS One 7, e50887 (2012).
Bai, X. L. et al. [Correlation of interleukin-10-1082G/a single nucleotide polymorphism to the risk of gastric cancer in north China: a case-control study]. Ai Zheng 27, 35–40 (2008).
Nieters, A. et al. Effect of cytokine genotypes on the hepatitis B virus-hepatocellular carcinoma association. Cancer 103, 740–748 (2005).
Lau, J., Ioannidis, J. P. & Schmid, C. H. Quantitative synthesis in systematic reviews. Ann Intern Med 127, 820–826 (1997).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Alpizar-Alpizar, W., Perez-Perez, G. I., Une, C., Cuenca, P. & Sierra, R. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med 5, 169–176 (2005).
Cacev, T., Radosevic, S., Krizanac, S. & Kapitanovic, S. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis 29, 1572–1580 (2008).
Cozar, J. M. et al. High incidence of CTLA-4 AA (CT60) polymorphism in renal cell cancer. Hum Immunol 68, 698–704 (2007).
Crivello, A. et al. Regulatory cytokine gene polymorphisms and risk of colorectal carcinoma. Ann N Y Acad Sci 1089, 98–103 (2006).
Crusius, J. B. et al. Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann Oncol 19, 1894–1902 (2008).
El-Omar, E. M. et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124, 1193–1201 (2003).
Forte, G. I. et al. Role of environmental and genetic factor interaction in age-related disease development: the gastric cancer paradigm. Rejuvenation Res 11, 509–512 (2008).
Garcia-Gonzalez, M. A. et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol 102, 1878–1892 (2007).
Guo, W. et al. Interleukin-10 -1082 promoter polymorphism is not associated with susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in a population of high-incidence region of north China. World J Gastroenterol 11, 858–862 (2005).
He, B. et al. Increased risk for gastric cancer in carriers of the lymphotoxin-alpha+252G variant infected by Helicobacter pylori. Genet Test Mol Biomarkers 16, 9–14 (2012).
Heneghan, M. A. et al. Frequency and nature of cytokine gene polymorphisms in hepatocellular carcinoma in Hong Kong Chinese. Int J Gastrointest Cancer 34, 19–26 (2003).
Kamangar, F. et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control 17, 117–125 (2006).
Kang, J. M. et al. The effects of genetic polymorphisms of IL-6, IL-8 and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol 43, 420–428 (2009).
Kim, J. et al. Effects of interleukin-10 polymorphisms, Helicobacter pylori infection and smoking on the risk of noncardia gastric cancer. PLoS One 7, e29643 (2012).
Ko, K. P. et al. Soybean product intake modifies the association between interleukin-10 genetic polymorphisms and gastric cancer risk. J Nutr 139, 1008–1012 (2009).
Lee, J. Y. et al. Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett 225, 207–214 (2005).
Lu, W. et al. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis 26, 631–636 (2005).
Macarthur, M., Sharp, L., Hold, G. L., Little, J. & El-Omar, E. M. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiol Biomarkers Prev 14, 1613–1618 (2005).
Migita, K. et al. Cytokine gene polymorphisms in Japanese patients with hepatitis B virus infection--association between TGF-beta1 polymorphisms and hepatocellular carcinoma. J Hepatol 42, 505–510 (2005).
Morgan, D. R. et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol 4, 1103–1111 (2006).
Ognjanovic, S., Yuan, J. M., Chaptman, A. K., Fan, Y. & Yu, M. C. Genetic polymorphisms in the cytokine genes and risk of hepatocellular carcinoma in low-risk non-Asians of USA. Carcinogenesis 30, 758–762 (2009).
Pan, X. F. et al. Interleukin-10 gene promoter polymorphisms and risk of gastric cancer in a Chinese population: single nucleotide and haplotype analyses. Asian Pac J Cancer Prev 14, 2577–2582 (2013).
Scola, L. et al. Genetic determined downregulation of both type 1 and type 2 cytokine pathways might be protective against pancreatic cancer. Ann N Y Acad Sci 1155, 284–288 (2009).
Shin, C. M. et al. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence and genetic susceptibility. Eur J Gastroenterol Hepatol 23, 411–417 (2011).
Shin, H. D. et al. Interleukin 10 haplotype associated with increased risk of hepatocellular carcinoma. Hum Mol Genet 12, 901–906 (2003).
Sugimoto, M. et al. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol 22, 1443–1449 (2007).
Wu, M. S. et al. Tumor necrosis factor-alpha and interleukin-10 promoter polymorphisms in Epstein-Barr virus-associated gastric carcinoma. J Infect Dis 185, 106–109 (2002).
Xiao, H., Jiang, Y., Li, R. & Xia, B. [Association of IL-10 gene polymorphisms with gastroduodenal diseases in Hubei Han population]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 26, 423–426 (2009).
Yin, Y. Q., Liu, C. J., Zhang, M. M. & Zhou, Y. [Interleukin-10-1082 promoter polymorphism and the risk of gastric cancer]. Sichuan Da Xue Xue Bao Yi Xue Ban 43, 378–381 (2012).
Zambon, C. F. et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 29, 141–152 (2005).
Zeng, X., Li, Y., Liu, T., Zhang, J. & Diverse, H. pylori strains, IL-10 promoter polymorphisms with high morbidity of gastric cancer in Hexi area of Gansu Province, China. Mol Cell Biochem 362, 241–248 (2012).
Zhou, Y., Hu, W., Zhuang, W. & Wu, X. Interleukin-10 -1082 promoter polymorphism and gastric cancer risk in a Chinese Han population. Mol Cell Biochem 347, 89–93 (2011).
Zhou, S. Z., Zhu, W. L., Li, M. Y., Li, H. Y. & Zhang, J. R. [Association of single nucleotide polymorphism at interleukin-10 gene 1082 nt with the risk of gastric cancer in Chinese population]. Nan Fang Yi Ke Da Xue Xue Bao 28, 1335–1338 (2008).
Aggarwal, B. B., Shishodia, S., Sandur, S. K., Pandey, M. K. & Sethi, G. Inflammation and cancer: how hot is the link? Biochem Pharmacol 72, 1605–1621 (2006).
Acknowledgements
This work has no funding.
Author information
Authors and Affiliations
Contributions
C.L., W.T. and B.L. designed this study; A.Z. and F.L. searched databases and collected full-text papers; C.L., W.T. and B.L., extracted and analyzed data; C.L., W.T., B.L., A.Z. and F.L. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Li, C., Tong, W., Liu, B. et al. The -1082A>G polymorphism in promoter region of interleukin-10 and risk of digestive cancer: a meta-analysis. Sci Rep 4, 5335 (2014). https://doi.org/10.1038/srep05335
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05335
This article is cited by
-
Association of IL-10 gene promoter polymorphisms with susceptibility to pseudoexfoliation syndrome, pseudoexfoliative and primary open-angle glaucoma
BMC Medical Genetics (2020)
-
Protective effects of Xinji′erkang on myocardial infarction induced cardiac injury in mice
BMC Complementary and Alternative Medicine (2017)
-
A polymorphism in the promoter region of PD-L1 serves as a binding-site for SP1 and is associated with PD-L1 overexpression and increased occurrence of gastric cancer
Cancer Immunology, Immunotherapy (2017)
-
The association of three promoter polymorphisms in interleukin-10 gene with the risk for colorectal cancer and hepatocellular carcinoma: A meta-analysis
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.