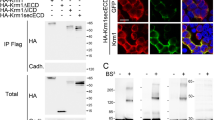
Abstract
Nogo-A is a potent neurite outgrowth inhibitory protein in vitro and is suggested to play a role in the lack of regeneration in the central nervous system of adult higher vertebrates. A shorter splice isoform, ASY/Nogo-B, has recently been reported to act as a proapoptotic protein, the loss of which would be typical for cancer cells. Here, we show that the osteosarcoma cell line SaOS-2 and the cell line CHO do express high levels of endogenous Nogo-B and that stable transfectants overexpressing high levels of Nogo-B do not differ significantly from the respective parental wild-type or control cell lines both in respect to cell proliferation and to spontaneous apoptosis or cell death induced by staurosporine and tunicamycin. The deletion of the second transmembrane domain of Nogo-B, which has been claimed to abolish its proapoptotic activity, leads to a shift of the protein from the ER to a cytoplasmic localization, suggesting that ER stress of highly overexpressed Nogo-B may lead to aversive cellular reactions under particular conditions. Our data do not support a function of Nogo-B as a physiological pro-apoptotic protein in certain types of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Adams JM and Cory S . (1998). Science, 281, 1322–1326.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W and Lipman DJ . (1997). Nucleic Acids Res., 25, 3389–3402.
Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B and Andrews DW . (2001). Oncogene, 20, 1939–1952.
Bongarzone ER, Jacobs E, Schonmann V, Kampf K, Campagnoni CW and Campagnoni AT . (2001). J. Neurosci. Res., 65, 485–492.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F and Schwab ME . (2000). Nature, 403, 434–439.
Cory S . (1995). Ann. Rev. Immunol., 13, 513–543.
Gross A, McDonnell JM and Korsmeyer SJ . (1999). Genes Dev., 13, 1899–1911.
Hacki J, Egger L, Monney L, Conus S, Rosse T, Fellay I and Borner C . (2000). Oncogene, 19, 2286–2295.
Herr I and Debatin KM . (2001). Blood, 98, 2603–2614.
Huber AB, Weinmann O, Brosamle C, Oertle T and Schwab ME . (2002). J. Neurosci., 22, 3553–3567.
Li Q, Qi B, Oka K, Shimakage M, Yoshioka N, Inoue H, Hakura A, Kodama K, Stanbridge EJ and Yutsudo M . (2001). Oncogene, 20, 3929–3936.
Michel A, Fischer U and Meese E . (1999). Electron. J. Pathol. Histol., 5, 993–997.
Morris NJ, Ross SA, Neveu JM, Lane WS and Lienhard GE . (1999). Biochim. Biophysic. Acta, 1450, 68–76.
Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N and Ohara O . (1998). DNA Res., 5, 355–364.
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA and Yuan J . (2000). Nature, 403, 98.
Nilsson I, Saaf A, Whitley P, Gafvelin G, Waller C and von Heijne G . (1998). J. Mol. Biol., 284, 1165–1175.
Oertle T, Huber C, van der Putten H and Schwab ME . (2003). J. Mol. Biol., 325, 299–323.
Pahl HL . (1999). Physiol. Rev., 79, 683–701.
Senden N, Linnoila I, Timmer E, van de Velde WJ, Roebroek A, Van de Ven WJ, Broers J and Ramaekers F . (1997a). Histochem. Cell Biol., 108, 155–165.
Senden NH, Timmer ED, Boers JE, Van de Ven WJ, Roebroek AJ, Broers JL and Ramaekers FC . (1996). Eur. J. Cell Biol., 69, 197–213.
Senden NH, Timmer ED, de Bruine A, Wagenaar SS, Van de Ven WJ, Roebroek AJ, Broers JL and Ramaekers FC . (1997b). J. Pathol., 182, 13–21.
Senden NH, Van de Ven WJ, Broers JL, Timmer ED, Kuijpers HJ, Roebroek AJ and Ramaekers FC . (1994). Eur. J. Cell Biol., 65, 341–353.
Shi Y . (2002). Mol. Cell, 9, 459–470.
Steller H . (1995). Science, 267, 1445–1449.
Tagami S, Eguchi Y, Kinoshita M, Takeda M and Tsujimoto Y . (2000). Oncogene, 19, 5736–5746.
Thompson CB . (1995). Science, 267, 1456–1462.
van de Velde HJK, Roebroek AJ, Senden NH, Ramaekers FC and Van de Ven WJ . (1994a). J. Cell Sci., 107, 2403–2416.
van de Velde HJK, Senden NH, Roskams TA, Broers JL, Ramaekers FC and Roebroek AJ . (1994b). Cancer Res., 54, 4769–4776.
Woynarowska BA and Woynarowski JM . (2002). Biochim. Biophys. Acta, 1587, 309–317.
Acknowledgements
We thank Dr Marjo Simonen (Novartis Pharma AG, Senior Scientific Expert Laboratory in Molecular and Cellular Biology) for critically reading the manuscript and helpful discussions, Chantal Huber for excellent technical assistance and Roland Schöb for graphical assistance. Special thanks to Dr Burkhardt Seifert (Department of Biostatistics, ISPM, University Zurich) for exceptional assistance in statistical analyses.
This study was supported by the Swiss National Science Foundation (Grant No. 31-63633).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oertle, T., Merkler, D. & Schwab, M. Do cancer cells die because of Nogo-B?. Oncogene 22, 1390–1399 (2003). https://doi.org/10.1038/sj.onc.1206278
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206278