Abstract
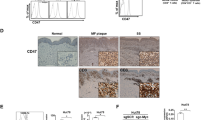
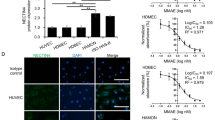
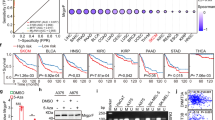
Biopsies from patients with cutaneous T-cell lymphoma (CTCL) exhibit stage-dependent increase in angiogenesis. However, the molecular mechanisms responsible for the increased angiogenesis are unknown. Here we show that malignant CTCL T cells spontaneously produce the potent angiogenic protein, vascular endothelial growth factor (VEGF). Dermal infiltrates of CTCL lesions show frequent and intense staining with anti-VEGF antibody, indicating a steady, high production of VEGF in vivo. Moreover, the VEGF production is associated with constitutive activity of Janus kinase 3 (Jak3) and the c-Jun N-terminal kinases (JNKs). Sp600125, an inhibitor of JNK activity and activator protein-1 (AP-1) binding to the VEGF promoter, downregulates the VEGF production without affecting Jak3 activity. Similarly, inhibitors of Jak3 inhibit the VEGF production without affecting JNK activity. Downregulation of Stat3 with small interfering RNA has no effect, whereas curcumin, an inhibitor of both Jak3 and the JNKs, almost completely blocks the VEGF production. In conclusion, we provide evidence of VEGF production in CTCL, which is promoted by aberrant activation of Jak3 and the JNKs. Inhibition of VEGF-inducing pathways or neutralization of VEGF itself could represent novel therapeutic modalities in CTCL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest 2005; 115: 798–812.
Foss F . Mycosis fungoides and the Sezary syndrome. Curr Opin Oncol 2004; 16: 421–428.
Vacca A, Moretti S, Ribatti D, Pellegrino A, Pimpinelli N, Bianchi B et al. Progression of mycosis fungoides is associated with changes in angiogenesis and expression of the matrix metalloproteinases 2 and 9. Eur J Cancer 1997; 33: 1685–1692.
Mazur G, Wozniak Z, Wrobel T, Maj J, Kuliczkowski K . Increased angiogenesis in cutaneous T-cell lymphomas. Pathol Oncol Res 2004; 10: 34–36.
Folkman J, Watson K, Ingber D, Hanahan D . Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989; 339: 58–61.
Ferrara N . Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25: 581–611.
Xie K, Wei D, Shi Q, Huang S . Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev 2004; 15: 297–324.
Pages G, Pouyssegur J . Transcriptional regulation of the vascular endothelial growth factor gene – a concert of activating factors. Cardiovasc Res 2005; 65: 564–573.
Kaltoft K, Bisballe S, Dyrberg T, Boel E, Rasmussen PB, Thestrup-Pedersen K . Establishment of two continuous T-cell strains from a single plaque of a patient with mycosis fungoides. In vitro Cell Dev Biol 1992; 28A: 161–167.
Davis TH, Morton CC, Miller-Cassman R, Balk SP, Kadin ME . Hodgkin's disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med 1992; 326: 1115–1122.
Wasik MA, Seldin DC, Butmarc JR, Gertz R, Marti R, Maslinski W et al. Analysis of IL-2, IL-4 and their receptors in clonally-related cell lines derived from a patient with a progressive cutaneous T-cell lymphoproliferative disorder. Leuk Lymphoma 1996; 23: 125–136.
Kaltoft K, Bisballe S, Rasmussen HF, Thestrup-Pedersen K, Thomsen K, Sterry W . A continuous T-cell line from a patient with Sezary syndrome. Arch Dermatol Res 1987; 279: 293–298.
Sommer VH, Clemmensen OJ, Nielsen O, Wasik M, Lovato P, Brender C et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia 2004; 18: 1288–1295.
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266: 11947–11954.
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res 2001; 61: 4143–4154.
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002; 21: 2000–2008.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16: 4604–4613.
Damert A, Ikeda E, Risau W . Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J 1997; 327 (Part 2): 419–423.
Nielsen M, Kaltoft K, Nordahl M, Ropke C, Geisler C, Mustelin T et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA 1997; 94: 6764–6769.
Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C et al. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia 2001; 15: 787–793.
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 2003; 22: 319–329.
Minet E, Michel G, Mottet D, Piret JP, Barbieux A, Raes M et al. c-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp Cell Res 2001; 265: 114–124.
Werlen G, Jacinto E, Xia Y, Karin M . Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J 1998; 17: 3101–3111.
Sharma RA, Gescher AJ, Steward WP . Curcumin: the story so far. Eur J Cancer 2005; 41: 1955–1968.
Chen YR, Tan TH . Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998; 17: 173–178.
Rajasingh J, Raikwar HP, Muthian G, Johnson C, Bright JJ . Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK–STAT pathway in T cell leukemia. Biochem Biophys Res Commun 2006; 340: 359–368.
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–732.
Ruiz M, Pettaway C, Song R, Stoeltzing O, Ellis L, Bar-Eli M . Activator protein 2alpha inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Res 2004; 64: 631–638.
Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN et al. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin Cancer Res 2005; 11: 267–272.
Pages G, Berra E, Milanini J, Levy AP, Pouyssegur J . Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J Biol Chem 2000; 275: 26484–26491.
Nielsen M, Nissen MH, Gerwien J, Zocca MB, Rasmussen HM, Nakajima K et al. Spontaneous interleukin-5 production in cutaneous T-cell lymphoma lines is mediated by constitutively activated Stat3. Blood 2002; 99: 973–977.
Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA . STAT3 induces transcription of DNA methyltransferase 1 (DNMT1) gene in malignant T-lymphocytes. Blood 2006; 108: 1058–1064.
Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene 2005; 24: 3110–3120.
Karjalainen JM, Kellokoski JK, Eskelinen MJ, Alhava EM, Kosma VM . Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J Clin Oncol 1998; 16: 3584–3591.
Ruiz M, Troncoso P, Bruns C, Bar-Eli M . Activator protein 2alpha transcription factor expression is associated with luminal differentiation and is lost in prostate cancer. Clin Cancer Res 2001; 7: 4086–4095.
Gee JM, Robertson JF, Ellis IO, Nicholson RI, Hurst HC . Immunohistochemical analysis reveals a tumour suppressor-like role for the transcription factor AP-2 in invasive breast cancer. J Pathol 1999; 189: 514–520.
Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM et al. Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas; comparison of immunohistochemistry and in situ hybridisation. J Clin Pathol 2001; 54: 533–538.
Nyormoi O, Bar-Eli M . Transcriptional regulation of metastasis-related genes in human melanoma. Clin Exp Metast 2003; 20: 251–263.
Gershenwald JE, Sumner W, Calderone T, Wang Z, Huang S, Bar-Eli M . Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene 2001; 20: 3363–3375.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342.
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001; 21: 2895–2900.
Acknowledgements
This work was supported in part by The Danish Research Councils, The Danish Cancer Society, The Novo Nordic Foundation, Fabrikant Vilhelm Pedersen og Hustrus Mindelegat, Dansk Kræftsforsknings Fond, scholarship from the The Danish Cancer Society (T Krejsgaard), the Deutsche Forschungsgemeinschaft (KFO124) (JC Becker) and Købmand i Odense Johann og Hanne Weimann f. Seedroffs Legat (T Labuda). We express our appreciation to Claudia Siedel for her excellent technical assistance and to Eva-Bettina Bröcker for critical discussions during the evaluation of the immunohistochemistry slides. We also thank K Kaltoft for providing us with the MyLa cell lines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Krejsgaard, T., Vetter-Kauczok, C., Woetmann, A. et al. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia 20, 1759–1766 (2006). https://doi.org/10.1038/sj.leu.2404350
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404350
Keywords
This article is cited by
-
The JAK/STAT signaling pathway: from bench to clinic
Signal Transduction and Targeted Therapy (2021)
-
Elucidating the mechanism of action of domatinostat (4SC-202) in cutaneous T cell lymphoma cells
Journal of Hematology & Oncology (2019)
-
Malignant T cells activate endothelial cells via IL-17 F
Blood Cancer Journal (2017)
-
Malignant inflammation in cutaneous T‐cell lymphoma—a hostile takeover
Seminars in Immunopathology (2017)
-
Sézary Syndrome: Clinical and Biological Aspects
Current Hematologic Malignancy Reports (2016)