Abstract
Advanced biliary tract carcinomas (BTCs) are often diagnosed at an advanced/metastatic stage and have a poor prognosis. The combination of gemcitabine and oxaliplatin (GEMOX) has shown promising activity in this setting. This international phase II study evaluated the efficacy and safety of GEMOX as first-line therapy in patients with advanced BTCs. Eligible patients with previously untreated locally advanced or metastatic BTC received gemcitabine 1000 mg m−2 (day 1) and oxaliplatin 100 mg m−2 (day 2), every 2 weeks. Seventy patients were enroled; 72.9% had metastatic disease. Sixty-seven patients were treated. There were 10 confirmed partial responses (14.9%; 95% confidence interval (CI), 7.4–25.7%) in the treated population (RECIST). Twenty-four patients (35.8 %) had stable disease. The objective response rate was 20.5% in patients with non-gallbladder cancers (9/44 patients) and 4.3% in patients with gallbladder cancers (1/23). Median overall survival for the intent-to-treat population was 8.8 months (95% CI, 6.9–11.1%) and progression-free survival was 3.4 months (95% CI, 2.5–4.6%). Grade 3/4 toxicities included thrombocytopenia (14.9% of patients), alanine aminotransferase elevation (13.4%), anaemia (10.4%), neutropenia (11.9%) and pain (11.9%). In this study, GEMOX demonstrated activity in non-gallbladder carcinoma, but poor activity in gallbladder carcinoma. GEMOX is well tolerated in advanced BTCs.
Similar content being viewed by others
Main
Biliary tract carcinomas (BTCs), comprising gallbladder carcinoma (GBC) and intra- and extrahepatic cholangiocarcinoma (CC), are relatively rare in the United States and Europe (de Groen et al, 1999). For example, approximately 5000 cases of GBC and 2000–3000 cases of CC are diagnosed annually in the United States. There are marked geographical variations in the incidence of GBC, although it is consistently more common in women than in men (Lazcano-Ponce et al, 2001). The symptoms of BTC are non-specific and tumours have often reached an advanced stage at diagnosis. As such, the prognosis for patients with BTC is extremely poor: median survival is generally lesser than 6 months and estimated 1- and 2-year survival rates are 25 and 13%, respectively (Patel, 2002). Chemotherapy is a palliative treatment option for patients with advanced disease. Owing to the lack of randomised phase III studies, there is no standard chemotherapy for advanced BTC. One clinical trial has demonstrated the improved survival for chemotherapy (5-fluorouracil plus leucovorin with or without etoposide) vs best supportive care (Glimelius et al, 1996), although the ability of chemotherapy to prolong survival remains to be confirmed.
Tolerability is of major importance when selecting palliative treatment regimens. Gemcitabine has palliative benefits and is generally well tolerated as therapy for advanced pancreatic carcinoma (Heinemann, 2002). Gemcitabine is also widely used as palliative therapy for advanced BTCs because of histogenetic similarities between the pancreas and biliary tract (Scheithauer, 2002).
Gemcitabine has shown promising activity against advanced BTCs, with response rates (RRs) in the range of 12–35% when used in combination with agents such as 5-fluorouracil, cisplatin, mitomycin C, or capecitabine (Kornek et al, 2004; Alberts et al, 2005; Kim et al, 2006; Riechelmann et al, 2007; Lee et al, 2008). A recent randomised phase II study suggested that combination chemotherapy with gemcitabine and cisplatin may be more effective than gemcitabine alone (Valle et al, 2006). The overall RR (ORR) was 24.3% for combination therapy and 15.2% for gemcitabine alone (complete response (CR)+partial response (PR)+stable disease (SD) was 75.7 vs 57.6%, respectively). Time to progression was also longer in the combination group (8.0 months) than in the monotherapy group (5.5 months). A follow-up study has been initiated with adequate power to assess the potential survival benefit of adding cisplatin to gemcitabine.
Oxaliplatin was used as monotherapy in one phase II study as first-line treatment for patients with BTC. An objective RR of 20.6% was observed with an overall survival (OS) of 7 months (Androulakis et al, 2006). Preclinical studies have demonstrated antitumour activity for the combination of gemcitabine and oxaliplatin (GEMOX) in human leukaemia and colorectal cancer cell lines and provide the rationale for using this combination in clinical studies. An optimal sequence-dependent synergy is apparent, with exposure to gemcitabine first and oxaliplatin later (Faivre et al, 1999).
A French phase II study (conducted in two centres) showed that the GEMOX combination was active and well tolerated as first-line chemotherapy in 36 patients with advanced BTCs (André et al, 2004); ORR (without confirmed response for all patients) was 35.5% (95% confidence interval (CI), 18.7–52.3%), progression-free survival (PFS) was 5.7 months and OS was 15.4 months. We undertook the present international phase II study to evaluate the efficacy and tolerability of GEMOX as first-line chemotherapy in a larger group of patients with advanced BTCs.
Patients and methods
Eligibility criteria
Patients aged >18 years with histologically proven, locally advanced or metastatic carcinoma of the biliary tract (gallbladder, intrahepatic bile ducts, extrahepatic bile ducts and ampulla of Vater) were enroled in the study. Other eligibility criteria included: Eastern Cooperative Oncology Group performance status ⩽2; unidimensionally measurable disease (Therasse et al, 2000); no prior chemotherapy for advanced disease; and adequate haematological (absolute neutrophil count >1.5 × 109 l−1, platelets >100 × 109 l−1), renal (creatinine <1.5 × the upper limit of normal; ULN), and hepatic function (alanine aminotransferase <5 × ULN; bilirubin <2.5 × ULN). Patients with jaundice or evidence of bile duct obstruction and in whom the biliary tree could be decompressed by endoscopic percutaneous endoprosthesis, with a subsequent reduction in bilirubin to <2.5 × ULN, were also eligible.
Patients with prior malignancy or prior chemotherapy for advanced disease, central nervous system metastases or peripheral neuropathy grade ⩾2 were excluded from the study. Prior radiation therapy within 4 weeks of the first gemcitabine administration was not permitted. Women of childbearing potential were required to be neither pregnant nor breastfeeding and to be under active contraception.
The study was conducted in compliance with Good Clinical Practice. All patients provided written informed consent.
Treatment plan
Treatment consisted of gemcitabine 1000 mg m−2 as a 100-min i.v. infusion on day 1 followed by oxaliplatin 100 mg m−2 as a 2-h i.v. infusion on day 2. Cycles were repeated every 2 weeks. Treatment was continued until disease progression, unacceptable toxicity, patient withdrawal of consent, or treatment delay of more than 3 weeks.
Oxaliplatin was stopped altogether for grade 3 neurological symptoms but could be reintroduced upon recovery (to grade ⩽2). For paraesthesia without pain that persisted between cycles (lasting greater than 14 days), oxaliplatin was stopped until recovery, and then restarted at 75 mg m−2. For paraesthesia with pain or functional impairment that persisted for greater than 7 but fewer than 14 days, the dose of oxaliplatin was reduced to 75 mg m−2; if the pain or functional impairment persisted between cycles, oxaliplatin was stopped. In the event of pharyngolaryngeal dysaesthesia during an infusion, the duration of oxaliplatin infusion was extended to 6 h for that and for subsequent infusions.
For patients with a platelet count <75 × 109 l−1, treatment was delayed for up to 3 weeks to allow recovery, and the oxaliplatin dose was reduced by 15% in subsequent cycles. For grade 3/4 thrombocytopenia, neutropenia, mucositis, diarrhoea or asthenia, the dose of gemcitabine was reduced to 800 mg m−2 over 80 min and the dose of oxaliplatin was reduced to 85 mg m−2 over 2 h. If grade 3/4 toxicity developed after dose reduction, the patient could be withdrawn from the study. In the event of oxaliplatin discontinuation for any toxicity, gemcitabine could be continued at the same dose and same schedule (every 2 weeks).
Assessments and follow-up
Tumour response was evaluated after four treatment cycles and every four cycles thereafter using the Response Evaluation Criteria in Solid Tumours (RECIST) unidimensional criteria (Therasse et al, 2000). Objective responses were to be confirmed after at least 4 weeks. Tumour burden was assessed in both target and non-target lesions. Target lesions were defined as lesions that were measurable in at least one dimension, with the longest diameter ⩾20 mm using conventional techniques or the longest diameter ⩾10 mm measured using spiral computed tomographic (CT) scan. Computed tomographic and magnetic resonance imaging scans were the only accepted imaging techniques for the determination of response. Non-target lesions were defined as all other lesions and non-measurable lesions. The integrated response was derived from the investigator's assessment of target and non-target lesions, taking into account the appearance of new lesions in accordance with RECIST. For target lesions, SD was categorised as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), taking the the smallest sum of the longest diameter as reference since the treatment started; for non-target lesions, SD was categorised as neither CR or PD. Assignment to the SD category could only be made at least 6 weeks (two cycles) after the start of treatment. The best overall response was determined following a sequential review of all integrated responses recorded from the start of treatment until disease progression, with the last evaluation taken as the 30-day post-treatment follow-up. Sequential evaluations were compared, and the best response from each pair was compared with the subsequent pair to derive the best overall response.
For patients who discontinued treatment for reasons other than disease progression, tumour evaluations were performed every 2 months. Whenever possible, the follow-up was continued until death.
Safety evaluations were performed in the exposed population before administration of each cycle and toxicity was graded using National Cancer Institute Common Toxicity Criteria version 2.
End points and statistics
The primary objective of this study was to evaluate the efficacy (based on RR) of the GEMOX regimen as first-line therapy in patients with advanced BTCs and rejecting the treatment for further study, if it was found to be insufficiently active. The study employed a one-stage design (Fleming, 1982) incorporating the following assumptions: H0: RR ⩽20% and H1: RR ⩾35%. On the assumption that of 56 evaluable patients, using a significance level of 0.05 and a power of 80%, GEMOX would be declared insufficiently active if ⩽12 responses were observed, and declared active if >12 responses were observed. To be evaluable for response, patients were to receive a minimum of two cycles of GEMOX (ie, 6 weeks on study) and were to have had at least one post-baseline tumour assessment unless early progression occurred first, in which case, patients were considered evaluable for response. Assuming that 25% of patients would have non-measurable disease, a sample of 70 patients was required, so that a total of 56 patients attained the requirements of the evaluable patient population.
An exploratory analysis was conducted to evaluate RRs by tumour type (GBC and CC). Kaplan–Meier survival curves were computed for time-to-event variables. Progression-free survival was calculated from the date of first treatment administration to the earliest date of disease progression, death or data cutoff. Overall survival was calculated from the date of first treatment administration to the date of death. Safety was reported for all subjects who received at least one dose of study drug.
Results
Patient characteristics
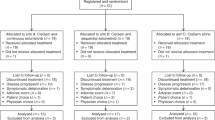
Between April 2003 and April 2005, 70 patients were enroled in the study, which was conducted in seven centres in France (2), Germany (2), Austria (1), Chile (1) and the United Kingdom (1). Patient and tumour characteristics at baseline are shown in Table 1.
Treatment received
Three patients did not receive study treatment: two died before starting treatment (one with GBC, one with CC) and one patient with CC had hyperbilirubinaemia. The exposed population, therefore, comprised 67 patients. In total, 479 cycles of oxaliplatin and 521 cycles of gemcitabine were administered. The median number of cycles was five for both oxaliplatin (range: 1–18 cycles) and gemcitabine (range: 1–30 cycles). Mean relative dose intensities were 94.1% for oxaliplatin and 98.4% for gemcitabine in the exposed population.
The primary reason for treatment discontinuation was disease progression (48 patients; 68.6%). Ten patients (14.3%) discontinued treatment as a result of adverse events (AEs), which were considered to be oxaliplatin-related hypersensitivity reactions in three patients (4.3%). Five patients continued treatment with gemcitabine alone after discontinuing oxaliplatin.
Efficacy
There were 10 PRs (14.9%;. 95% CI, 7.4–25.7%) in the exposed population (Table 2). A further five unconfirmed PRs were observed in the exposed population (three GBCs and three CCs). The majority of responses were observed in patients with CC: PRs were observed for 9/44 patients (20.5%) with CC and 1/23 patients (4.3%) with GBC.
Median PFS was 3.4 months (95% CI, 2.5–4.6 months) for both the ITT and exposed populations (Figure 1A). Median PFS was 2.5 months for patients with GBC (95% CI, 1.6–4.3 months) and 3.8 months for patients with CC (95% CI, 2.7–5.6 months; Figure 1B).
Overall, 59 deaths were reported during the study period in the ITT population, 57 of which were in the exposed population. One patient died 1 month after the study cutoff date, but was considered alive for the purpose of the survival analysis. Median OS was 8.8 months (95% CI, 6.9–11.1 months) in the ITT population and 9.3 months (95% CI, 6.9–11.4 months) in the exposed population (Figure 2A). For both populations, median OS was 11.0 months for patients with non-GBC and 6.1 months for patients with GBC (Figure 2B).
Safety
Sixty-seven patients received at least one administration of study treatment and were included in the safety analysis. There were six deaths during the treatment period (between cycle 1 and cycle 4), none of which was considered by the investigators to be treatment-related. However, after reviewing the patient data, the sponsor could not rule out a relationship to study treatment for three deaths; one patient developed septicaemia and had global status deterioration, one patient had diarrhoea and vomiting leading to the rapid health deterioration and respiratory arrest, and one patient died from general health deterioration.
Overall, nausea (82.1%) and vomiting (56.7%) of all grades were frequent side effects, despite systemic prophylactic measures (Table 3). Grade 3 nausea and vomiting occurred in 4.5% and 10.4% of patients, respectively, although there were no grade 4 events of this type. Overall, grade 3/4 AEs occurred in 47 patients (70.1%).
Peripheral sensory neuropathies were observed in 67.2% of patients, with grade 3 neuropathy in 6.0%, including one case of laryngospasm. No grade 4 neuropathy was reported. In general, neuropathies were mild (grade 1) and intermittent during the first treatment cycles but tended to worsen and become more persistent as the number of treatment cycles increased. Neurotoxicity grade ⩾2 occurred in 40% of patients who received ⩾9 treatment cycles. Other frequently reported AEs included anaemia (77.6%), fatigue (73.1%), thrombocytopenia (68.7%), liver enzyme increase (62.7%), and weight loss (61.2%), although the majority of these events were grade 1/2 in severity.
Discussion
This study is one among the largest of the phase II studies conducted to date to evaluate the efficacy and tolerability of chemotherapy in patients with advanced BTCs. The majority of patients enroled in this study had metastatic disease. The GEMOX administered every 2 weeks was well tolerated and conferred an ORR of 14.9%, with 50.7 % of patients achieving a PR or SD.
The ORR is somewhat lower than previously reported for GEMOX (André et al, 2004) or for gemcitabine in combination with other chemotherapy agents (Kim et al, 2006; Eckel and Schmid, 2007; Riechelmann et al, 2007; Lee et al, 2008). Our goal of achieving a 20% RR in this phase II study was not reached, when responses were assessed using RECIST. However, the percentage of combined confirmed and unconfirmed PR (23.9%) was more in line with other published phase II studies in advanced BTCs, the majority of which report RRs that are often unconfirmed, as opposed to using RECIST confirmed responses. In our study, most unconfirmed PRs were those that did not persist from one assessment to the next (tumour response evaluation every 2 months).
There is currently no standard chemotherapy regimen for advanced BTCs. Gemcitabine is one of the most widely used agents in this setting, with RRs of 12–35% for gemcitabine-based combination regimens, although these rates are unconfirmed in the majority of studies. A pooled analysis of studies (112 studies, including one phase III study; 2810 patients) performed between 1985 and 2006 demonstrated that the combination of gemcitabine with platinum compounds increased RRs and tumour-control rates compared with gemcitabine alone (Eckel and Schmid, 2007). Response rates of 22–50% have been observed in single-institution studies, with OS of 7.6–15.4 months (André et al, 2004; Harder et al, 2006; Verderame et al, 2006). However, single-institution studies often report higher RRs than multinational studies.
For this study, we conducted an experimental subgroup analysis to assess response to treatment in patients with GBC and CC. Our study population comprised approximately one-third of the patients with GBC, who are generally considered to have a worse prognosis than patients with other BTCs (Gallardo et al, 2005). In BTC, prognostic factors could be more important than therapy itself in determining treatment outcome. This study confirms the differential outcome between the gallbladder compared with other cancers of the biliary tree, as observed by Gallardo et al (2005).
In the pooled analysis by Eckel and Schmid (2007), the median RR for trials in patients with GBC was higher than that in patients with CC (35.5 vs 17.7%; P=0.008). However, the response duration is short for GBC, and OS was significantly longer in trials of patients with CC compared with GBC (median 9.3 vs 7.2 months; P=0.048) (Eckel and Schmid, 2007). In our study, RRs and RR+SD values in the GBC and CC subgroups were 4.0 vs 20.0% and 40 vs 53%, respectively. Concerning the survival, GBC was associated with a shorter PFS duration than CC (2.5 vs 3.8 months, respectively) and shorter median OS (6.1 vs 11.0 months, respectively). The reported efficacy,especially for GBC, differed markedly from a previous study of GEMOX in BTC, where the RR (unconfirmed) and OS were higher for GBC (n=11) vs CC (n=16; 54.4 vs 21.4%; 16.0 vs 14.5 months; André et al, 2004). The differences in the results of the two studies are surprising and could be related to the small number of patients, a centre effect, or the inclusion of unconfirmed responses.
The combination of gemcitabine and oxaliplatin was generally well tolerated. Grade 3/4 AEs were reported in 70.1% of patients, although all patients experienced at least one AE of any grade. As previously observed with the GEMOX combination (André et al, 2004), the most frequent grade 3/4 AEs were neutropenia, thrombocytopenia, pain and vomiting, all of which occurred in <15% of patients. Neutropenia and thrombocytopenia appear to occur less frequently with the GEMOX regimen than with the combination of gemcitabine and cisplatin (Park et al, 2006; Lee et al, 2008; Meyerhardt et al, 2008). Patients with advanced BTCs are very susceptible to infection and disease progression. Therefore, caution will be necessary with GEMOX chemotherapy like all chemotherapies in this disease. Grade 3 sensory neuropathy was uncommon (6.0%).
In conclusion, this multinational study provides further evidence for the activity of GEMOX as a treatment for non-GBC, but also demonstrates the poor activity of this agent in GBCs. The combination of gemcitabine and oxaliplatin is well tolerated and provides a treatment option for patients with advanced BTCs, and in particular non-GBCs. A phase III study comparing GEMOX to gemcitabine is necessary to further establish the role of GEMOX in advanced BTCs. The design of such a study should include stratification for the location of the carcinoma (non-GBCs vs GBCs).
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alberts SR, Al-Khatib H, Mahoney MR, Burgart L, Cera PJ, Flynn PJ, Finch TR, Levitt R, Windschitl HE, Knost JA, Tschetter LK (2005) Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 103: 111–118
André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, Gayet B, Lotz JP, de Gramont A, Louvet C, GERCOR Group (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15: 1339–1343
Androulakis N, Aravantinos G, Syrigos K, Polyzos A, Ziras N, Tselepatiotis E, Samonis G, Kentepozidis N, Giassas S, Vamvakas L, Georgoulias V (2006) Oxaliplatin as first-line treatment in inoperable biliary tract carcinoma: a multicentre phase II study. Oncology 70: 280–284
de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM (1999) Biliary tract cancers. N Engl J Med 341: 1368–1378
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96: 896–902
Faivre S, Raymond E, Woynarowski JM, Cvitkovic E (1999) Supraadditive effect of 2′,2′-difluorodeoxycytidine (gemcitabine) in combination with oxaliplatin in human cancer cell lines. Cancer Chemother Pharmacol 44: 117–123
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38: 143–151
Gallardo J, Rubio B, Villanueva L, Barajas O (2005) Gallbladder cancer, a different disease that needs individual trials. J Clin Oncol 23: 7753–7754
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7: 593–600
Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, Geissler M, Opitz O, Henss H (2006) Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 95: 848–852
Heinemann V (2002) Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. Semin Oncol 29 (Suppl 20): 9–16
Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, Heo JS, Park YS, Kang WK, Park K (2006) A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 106: 1339–1346
Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, Depisch D, Lang F, Scheithauer W (2004) Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised Phase II trial. Ann Oncol 15: 478–483
Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista G, Nervi F (2001) Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 51: 349–364
Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, Lee NS, Park BJ, Kim JS, on behalf of the Korean Cancer Study Group (2008) Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 61: 47–52
Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, Earle CC, Kulke MH, Bhargava P, Fuchs CS (2008) Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci 53: 564–570
Park BK, Kim YJ, Park JY, Bang S, Park SW, Chung JB, Kim KS, Choi JS, Lee WJ, Song SY (2006) Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. J Gastroenterol Hepatol 21: 999–1003
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2: 10
Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ (2007) Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 110: 1307–1312
Scheithauer W (2002) Review of gemcitabine in biliary tract carcinoma. Semin Oncol 29 (Suppl 20): 40–45
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumours. J Natl Cancer Inst 92: 205–216
Valle JW, Wasan H, Johnson P, Bridgewater J, Maraveyas A, Jones E, Tunney V, Swindell R, on behalf of the ABC-01 study group (2006) Gemcitabine, alone or in combination with cisplatin, in patients with advanced or metastatic cholangiocarcinoma (CC) and other biliary tract tumours: a multicentre, randomized, phase II (the UK ABC-01) study. ASCO Gastrointestinal Cancer Symposium abstract no. 98
Verderame F, Russo A, Di Leo R, Badalamenti G, Santangelo D, Cicero G, Valerio MR, Gulotta G, Tomasello G, Gebbia N, Fulfaro F (2006) Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol 17 (Suppl 7): vii68–vii72
Acknowledgements
The study was sponsored by sanofi-aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
André, T., Reyes-Vidal, J., Fartoux, L. et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 99, 862–867 (2008). https://doi.org/10.1038/sj.bjc.6604628
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604628
Keywords
This article is cited by
-
Current Standards, Multidisciplinary Approaches, and Future Directions in the Management of Extrahepatic Cholangiocarcinoma
Current Treatment Options in Oncology (2024)
-
Triple therapy in biliary tract cancers: GemOX plus immune checkpoint inhibitor in combination with lenvatinib or NGS-guided targeted therapy
Journal of Cancer Research and Clinical Oncology (2023)
-
Optimizing Patient Pathways in Advanced Biliary Tract Cancers: Recent Advances and a French Perspective
Targeted Oncology (2023)
-
Gemcitabine, Cisplatin, and Nab-Paclitaxel as a First-Line Therapy for Advanced Biliary Tract Cancers
Journal of Gastrointestinal Cancer (2023)
-
Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma
Scientific Reports (2021)