Abstract
Concurrent chemoradiation therapy (CCRT) is the treatment of choice for locally advanced non-small cell lung cancer (LA-NSCLC). Several clinical trials that combine programmed cell death 1 (PD-1) axis inhibitors with radiotherapy are in development for patients with LA-NSCLC. However, the effect of CCRT on programmed cell death ligand-1 (PD-L1) expression on tumor cells is unknown. In this study, we analysed paired NSCLC specimens that had been obtained pre- and post-CCRT. PD-L1 expression on tumor cells was studied by immunohistochemistry. A total of 45 patients with LA-NSCLC were included, among which there were sufficient pre- and post-CCRT specimens in 35 patients. Overall, the percentage of tumor cells with PD-L1 expression significantly decreased between pre- and post-CCRT specimens (P = 0.024). Sixteen, 15, and 4 patients had decreased, unchanged, or increased PD-L1 expression after CCRT, respectively. Median OS of patients with decreased, unchanged, or increased PD-L1 expression was 85.1, 92.8, and 14.6 months, respectively (P < 0.001). In conclusion, the percentage of PD-L1-positive tumor cells significantly decreased after CCRT. Alteration of PD-L1 expression after neoadjuvant CCRT was associated with prognosis in patients with LA-NSCLC. These data should be considered when developing the optimal approach of integrating PD-1 axis inhibitors with CCRT.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide1. Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of lung cancers, and concurrent chemoradiation therapy (CCRT) is the treatment of choice for locally advanced NSCLC (LA-NSCLC)2. However, the prognosis of LA-NSCLC remains poor, despite recent efforts to improve outcomes via the use of new cytotoxic drugs or high-dose radiotherapy2,3,4.
Recently, programmed cell death 1 (PD-1)/programmed cell death ligand-1 (PD-L1) checkpoint inhibitors demonstrated impressive anti-tumor activity for the treatment of metastatic NSCLC5,6,7,8,9. Thus, there is substantial interest in extending the benefit of these inhibitors to LA-NSCLC patients. Although there are limited data on the efficacy of combining radiation therapy and immunotherapy, this combination has the ability to achieve a synergistic therapeutic effect10,11,12. Several clinical trials that combine these agents with radiotherapy in patients with LA-NSCLC are in the planning stage13, 14. Therefore, we need the optimal approach to integrate PD-1 axis inhibitors with CCRT.
In most trials of PD-1 axis inhibitors for metastatic NSCLC, immunohistochemical (IHC) analysis of PD-L1 expression has been used as a predictive diagnostic test to identify responders and to guide treatment in trials of PD-1 axis inhibitors in NSCLC patients5,6,7,8,9. Particularly, in a recent first-line trial, pembrolizumab was associated with significantly longer progression-free and overall survival than platinum-based chemotherapy in patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells. Thus, high PD-L1 expression is a potential good predictive biomarker for the efficacy of PD-1 axis inhibitors. Despite the successful use of PD-L1 expression in the trial, there were several problems in assessing PD-L1 expression. One problem was that the expression of PD-L1 on tumor cells was not consistent. Anti-cancer systemic therapy influenced PD-L1 expression on tumor cells in previous reports15, 16. However, the effect of CCRT on PD-L1 expression on tumor cells is not known.
The aims of this study were to analyse paired NSCLC specimens that had been obtained pre- and post-CCRT to explore the impact of CCRT on PD-L1 expression and to suggest possible optimal approaches of integrating PD-1 axis inhibitors with CCRT.
Results
Patient characteristics
A total of 45 LA-NSCLC patients with sufficient specimens before CCRT were included in this study (Fig. 1). All patients were treated with CCRT followed by surgery. Patient characteristics are summarized in Table 1. The median time between the last induction day of CCRT and the surgery was 32 days (interquartile range, 29–36 days). Fourteen patients had stage II disease, while 31 patients had stage III disease. The majority of patients (67%) received vinorelbine plus platinum as the CCRT regimen. Only 4 patients received adjuvant chemotherapy after the surgery. Twenty-eight (62%) had positive PD-L1 expression on tumor cells in the pre-CCRT specimens.
PD-L1 status and CD 8+ lymphocytes density between matched pre- and post-CCRT specimens
Among the 45 patients with sufficient material before CCRT, there were 35 patients in whom there was sufficient material with tumor cells both before and after CCRT (Table 2). Among the 35 patients, 22 patients (63%, 22/35) had PD-L1 expression on tumor cells in the pre-CCRT specimens, and 21 patients (60%, 21/35) had PD-L1 expression on tumor cells in the post-CCRT specimens. Overall, the percentage of tumor cells with PD-L1 expression significantly decreased between the pre- and post-CCRT specimens (P = 0.024) (Fig. 2a and b). Sixteen, 15, and 4 patients had decreased, unchanged, or increased PD-L1 expression after CCRT compared with that before CCRT, respectively. Of the 15 patients with unchanged PD-L1 expression, PD-L1 expression was negative in both the pre-CCRT specimens and it remained negative in the post-CCRT specimens in 11 patients (73%, 11/15).
We compared the clinical profiles of the patients with decreased (n = 16) or non-decreased PD-L1 expression (n = 19) after CCRT. The patients with decreased PD-L1 expression included a significantly lower proportion of patients with increased CD8+ lymphocytes density (3/16 vs. 14/18, respectively, P < 0.001; one patient with non-decreased PD-L1 expression did not have sufficient material with stroma) (Table 3).
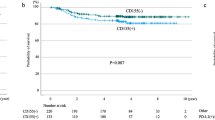
We also investigated the changes in stromal CD8+ lymphocytes density after CCRT. Sufficient material with stroma and tumor cells before CCRT was available in 43 of the 45 patients. Further, we had sufficient material with stroma and tumor cells both before and after CCRT in 34 of the 35 patients; there was not enough material with stroma in the pre-CCRT specimens of one patient. Among the 34 patients with sufficient material with tumor cells and stroma before and after CCRT, one, 16, or 17 patients had decreased, unchanged, or increased stromal CD8+ lymphocytes after CCRT, respectively (Fig. 2c).
Association of PD-L1 expression with survival time
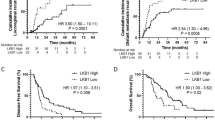
Among the 45 patients with sufficient pre-CCRT material with tumor cells, PD-L1 expression in pre-CCRT material was not significantly associated with RFS (PD-L1-positive group versus PD-L1-negative group, median 28.8 versus 27.9 months, p = 0.546, respectively) and OS (PD-L1-positive group versus PD-L1-negative group, median 94.1 versus 92.8 months, p = 0.746, respectively). Among the 35 patients with sufficient post-CCRT tumor material, PD-L1 expression in post-CCRT material was not significantly associated with RFS (PD-L1-positive group versus PD-L1-negative group, median 21.9 versus 22.4 months, p = 0.939, respectively) and OS (PD-L1-positive group versus PD-L1-negative group, median 85.1 versus 73.9 months, p = 0.784, respectively). These survival curves are shown in Fig. 3.
Kaplan–Meier survival curves. Kaplan–Meier survival curves of overall survival in patients with or without PD-L1 expression on tumor cells in the pre-CCRT specimens (a), or post-CCRT specimens (b). Kaplan–Meier survival curves of recurrence-free survival in patients with or without PD-L1 expression on tumor cells in the pre-CCRT specimens (c), or post-CCRT specimens (d).
We also investigated the association between the change in PD-L1 expression and survival time. The median RFS of patients with decreased, unchanged, or increased PD-L1expression was 32.0, 17.6, and 8.7 months, respectively (P = 0.079). In addition, the median OS of patients with decreased, unchanged, or increased PD-L1expression was 85.1, 92.8, and 14.6 months, respectively (P < 0.001). These survival curves are shown in Fig. 4. No patient received PD-1 axis inhibitors during the observation period.
Association of CD8+ lymphocytes density with survival time
The patients with intermediate-high CD8+ lymphocytes density in the pre-CCRT material tended to have longer RFS than those with low CD8+ lymphocytes density in the pre-CCRT material (intermediate-high group versus low group of pre-CCRT material, median 94.0 versus 19.5 months, p = 0.127). The patients with intermediate-high CD8+ lymphocytes density in the post-CCRT material also tended to have longer RFS (intermediate-high group versus low group of post-CCRT material, median 27.1 versus 11.2 months, p = 0.093). In addition, there was a similar trend between CD8+ lymphocytes density and OS in patients with pre-CCRT material (intermediate-high group versus low group of pre-CCRT material, median not reached versus 77.4 months, p = 0.232) and patients with post-CCRT material (intermediate-high group versus low group of post-CCRT material, median 92.8 versus 35.6 months, p = 0.101). The change in CD8+ lymphocytes density was not significantly associated with RFS (increased group versus unchanged or decreased group, median 19.5 versus 21.9 months, p = 0.435, respectively) and OS (increased group versus unchanged or decreased group, median 92.8 versus 58.5 months, p = 0.786, respectively). These survival curves are shown in Supplementary Figure 2 and Supplementary Figure 3.
Discussion
To the best of our knowledge, this study provides the first report of alteration of PD-L1 expression after neoadjuvant CCRT and its prognostic impact in patients with LA-NSCLC.
Our results indicated that the percentage of PD-L1-positive tumor cells significantly decreased after CCRT. Patients with increased PD-L1 expression after CCRT were relatively rare (4/35) in our study. A recent study also demonstrated that chemotherapy significantly reduced PD-L1 expression on tumor cells in NSCLC patients15. When we considered these results, CCRT can reduce PD-L1 expression on tumor cells in many patients.
In our study, we could not demonstrate the prognostic value of the baseline PD-L1 expression status, which still remains controversial in lung cancer17. Although we additionally analysed this prognostic association under the positive cut-off value of 5% of tumor cells, we could not demonstrate the prognostic value of the baseline PD-L1 expression status (data not shown). However, the change in PD-L1 expression after CCRT was associated with survival. Especially, increased PD-L1 expression was related to poor survival, although the proportion of patients with increased PD-L1 expression was small. A similar trend was reported in the neoadjuvant chemotherapy setting15, 18. The precise reason for this association was unclear. One possible explanation is the malignant feature of tumor cells with increased PD-L1 expression after anti-cancer treatment. One study reported that increased PD-L1 expression promoted the resistant response in lung cancer cells18. Another study indicated that high PD-L1 expression was related to high proliferative activity and the epithelial-mesenchymal transition phenotype19. The present study was too small to be able to draw firm conclusions. However, when we considered these previous results with ours, there seems to be an association between the change in PD-L1 expression and prognosis.
We could not find predictive factors associated with the change in PD-L1 expression in our study. Especially, the expression profile was not correlated with the chemotherapy regimen, although previous reports indicated the association in vitro in lung cancer tumor cells20, 21. There was a discrepancy in the effect of chemotherapy on PD-L1 regulation among different malignancies and agents22,23,24,25. In addition, a recent study demonstrated that chemotherapy decreased PD-L1 expression in clinical specimens of gastrointestinal cancers, although the same chemotherapy upregulated PD-L1 expression in vitro on tumor cells26. Therefore, future studies on this association using clinical lung cancer specimens are necessary.
We also found that the stromal CD8+ lymphocytes density increased after CCRT. We observed that patients with intermediate-high stromal CD8+ lymphocytes density in the pre- or post-CCRT material tended to have longer RFS and OS, similar to previous studies27, 28. However, the change in CD8+ lymphocytes density after CCRT was not associated with survival time. Interestingly, the patients with decreased PD-L1 expression included a significantly lower proportion of patients with increased CD8+ lymphocytes density. Increased PD-L1 expression and increased number of tumor-infiltrating lymphocytes were associated with better response to PD-1 axis inhibitors9, 29, 30. Therefore, these patients might have a good response to PD-1 axis inhibitors after CCRT.
Our study demonstrated the alteration of PD-L1 expression and its prognostic impact after CCRT in NSCLC patients. However, we did not investigate other immune checkpoint molecules such as PD-1 and cytotoxic T lymphocyte antigen-4 (CTLA-4) in our study. The up- and down-modulation of these molecules after radiation have also been reported in some studies31, 32. In a previous study in cervical cancer patients, elevated PD-1 expression on CD4+ T cells was demonstrated after CCRT33. Another study on rectal cancer indicated that CTLA-4 expression was relatively stable after chemoradiation therapy34. Actually, several small studies suggested the clinical efficacy of CTLA-4 inhibitor with radiation in several malignancies35,36,37. Although the prognostic importance of these molecules was not confirmed in clinical lung cancer studies, further research is warranted to study the association between chemoradiation and the change in the expression of these molecules in clinical specimens.
Several clinical trials that combine immune check point inhibitors with radiotherapy are in development for patients with LA-NSCLC13, 14. Therefore, we need the optimal approach to integrate PD-1 axis inhibitors with CCRT. Unfortunately, none of the patients in our cohort received PD-1/PD-L1 inhibitors after CCRT or combination therapy of PD-1/PD-L1 inhibitors and radiation. Therefore, these clinical studies are warranted in the future. We speculate two approaches in the treatment of LA-NSCLC, when we considered the results of our study. One approach is to administer PD-1 axis inhibitors before CCRT, because CCRT generally reduced PD-L1 expression. Another approach is to administer these agents after CCRT, because patients with increased PD-L1 expression had poor prognosis. In addition, patients with increased PD-L1 expression had increased CD8+ lymphocytes. Therefore, we might improve the prognosis of these poor prognostic patients by administering PD-1 axis inhibitors after CCRT.
This study had several limitations. First, there was the potential for selection bias, because this study was conducted at a single institution and we included patients who underwent surgery. Second, this study was limited by the relatively small sample size. However, even though the sample size was small, the main outcome was statistically significant. Therefore, we believe that this study provided evidence of decreased PD-L1 expression after CCRT and its associated increase in OS. Third, the chemotherapy regimen was determined by each doctor and therefore differed among the patients. Fourth, we did not investigate PD-L1 expression with another method such as fluorescent in situ hybridization (FISH).
In conclusion, CCRT significantly reduced the percentage of tumor cells with PD-L1 expression. Alteration of PD-L1 expression after neoadjuvant CCRT was associated with prognosis in patients with LA-NSCLC. We should consider these data to determine the optimal approach of integrating PD-1 axis inhibitors with CCRT in order to improve the prognosis of these patients.
Methods
Patients
We retrospectively analysed patients who were diagnosed with LA-NSCLC and who underwent CCRT followed by surgery (complete resection) at Kobe City Medical Center General Hospital between January 2004 and January 2013 (Fig. 1). We included patients in whom we had sufficient specimens with tumor cells before CCRT for analysis. This study included patients with stage II to III disease of LA-NSCLC who underwent surgery as part of a multimodality treatment approach38. Patients who reported never having smoked were defined as never smokers, those who had smoked within 1 year of the diagnosis were categorized as current smokers and the rest were considered to be former smokers. All patients were classified on the basis of clinical stage according to the 7th edition TNM classification39. The post-CCRT specimens were obtained at the time of surgical resection. Recurrence-free survival (RFS) was defined as the period from the day of surgery until any recurrence of lung cancer or death from any cause or the end of the follow-up. Overall survival (OS) was defined as the period from the day of surgery until death from any cause or the end of the follow-up. The observation period was until August 2016. This study was approved by the Kobe City Medical Center General Hospital Ethics Committee. All methods were performed in accordance with the 1975 Declaration of Helsinki. All tumor specimens in the pathological analysis were obtained with informed consent (or formal waiver of consent) with approval by the Ethics Committee of our hospital.
Evaluation of PD-L1 expression and stromal CD8-positive T cells
Histological samples were defined as sufficient specimens if ≥100 tumor cells were present. The expression of PD-L1 in human NSCLC specimens was analysed by IHC staining using rabbit monoclonal anti-human antibody (clone 28-8, Abcam, 1:150). Four-μ m-thick sections were cut from the formalin-fixed, paraffin-embedded tumor block and then routinely deparaffinized and rehydrated. The antibody was applied according to the DAKO-recommended detection methods and previous reports40,41,42. A placenta specimen served as the positive control (Supplementary Fig. 1). The scoring of PD-L1 in tumor cells was expressed as a percentage of stained cells in the overall section of tumor and estimated in increments of 5% except for 1% positivity of tumor cells. Patients with 1% or greater PD-L1 staining of tumor cells were considered to be positive for PD-L1 expression, consistent with previous studies using this antibody5,6,7. Patients with positive PD-L1 expression were divided into two groups according to the main criteria commonly used in clinical NSCLC cases: weakly positive (percentage of stained cells of 1–49%), or strongly positive (percentage of stained cells of ≥50%)43. Unchanged PD-L1 expression was defined as the same percentage of stained cells in the specimens obtained before and after CCRT from the same patient.
The presence of stromal CD8-positive lymphocytes in NSCLC specimens was assessed with IHC staining using prediluted primary CD8 antibody from DAKO (C8/144B). The percentage of CD8-positive lymphocytes compared with the total amount of nucleated cells in the stromal compartments was assessed. The following cutoffs were used according to previous reports44, 45: low density ≤25%; intermediate density >25% to 50%; high density: >50%. An increase in CD8-positive lymphocytes was defined as a change from low density to intermediate or high density, or from intermediate to high density after CCRT.
Samples were anonymized and independently scored by two pathologists (K.U. and Y.I.). In cases of disagreement, the slides were re-examined and the two pathologists reached a consensus.
Statistical analysis
Continuous variables were analysed using Student’s t-test. Dichotomous variables were analysed using the χ2 or Fisher’s exact test, as appropriate. The change in PD-L1 positivity status was analysed using Wilcoxon signed-rank test. The Kaplan–Meier method was used to estimate the survival outcomes and groups were compared using the log-rank test. The results are expressed as hazard ratios (HRs) with 95% confidence interval (CI). A P-value of < 0.05 was considered to indicate statistical significance. We conducted statistical analyses using JMP 9 software (SAS Institute, Cary, NC, USA).
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA: a cancer journal for clinicians 66, 7–30, https://doi.org/10.3322/caac.21332 (2016).
Auperin, A. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 2181–2190, https://doi.org/10.1200/JCO.2009.26.2543 (2010).
Bradley, J. D. et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet. Oncology 16, 187–199, https://doi.org/10.1016/S1470-2045(14)71207-0 (2015).
Senan, S. et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 34, 953–962, https://doi.org/10.1200/JCO.2015.64.8824 (2016).
Borghaei, H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 373, 1627–1639, https://doi.org/10.1056/NEJMoa1507643 (2015).
Brahmer, J. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine 373, 123–135, https://doi.org/10.1056/NEJMoa1504627 (2015).
Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550, https://doi.org/10.1016/S0140-6736(15)01281-7 (2016).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846, https://doi.org/10.1016/S0140-6736(16)00587-0 (2016).
Reck, M. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine, doi:https://doi.org/10.1056/NEJMoa1606774 (2016).
Domagala-Kulawik, J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Translational lung cancer research 4, 177–190, https://doi.org/10.3978/j.issn.2218-6751.2015.01.11 (2015).
Tang, C. et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer immunology research 2, 831–838, https://doi.org/10.1158/2326-6066.CIR-14-0069 (2014).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer research 74, 5458–5468, https://doi.org/10.1158/0008-5472.CAN-14-1258 (2014).
Daly, M. E., Monjazeb, A. M. & Kelly, K. Clinical Trials Integrating Immunotherapy and Radiation for Non-Small-Cell Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 10, 1685–1693, https://doi.org/10.1097/JTO.0000000000000686 (2015).
Hanna, N. Current Standards and Clinical Trials in Systemic Therapy for Stage III Lung Cancer: What Is New? American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Meeting, e442–447, doi:https://doi.org/10.14694/EdBook_AM.2015.35.e442 (2015).
Sheng, J. et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Scientific reports 6, 20090, https://doi.org/10.1038/srep20090 (2016).
Gainor, J. F. et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clinical cancer research: an official journal of the American Association for Cancer Research 22, 4585–4593, https://doi.org/10.1158/1078-0432.CCR-15-3101 (2016).
Mino-Kenudson, M. Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer biology & medicine 13, 157–170, https://doi.org/10.20892/j.issn.2095-3941.2016.0009 (2016).
Zhang, P. et al. The up-regulation of PD-L1 promotes the resistant response in non-small cell lung cancer patients with neo-adjuvant chemotherapy. Cancer science, doi:https://doi.org/10.1111/cas.13072 (2016).
Shimoji, M. et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer 98, 69–75, https://doi.org/10.1016/j.lungcan.2016.04.021 (2016).
Chen, N. et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 10, 910–923, https://doi.org/10.1097/JTO.0000000000000500 (2015).
Zhang, P. et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer science 107, 1563–1571, https://doi.org/10.1111/cas.13072 (2016).
Jiang, X., Zhou, J., Giobbie-Hurder, A., Wargo, J. & Hodi, F. S. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research 19, 598–609, https://doi.org/10.1158/1078-0432.CCR-12-2731 (2013).
Peng, J. et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer research 75, 5034–5045, https://doi.org/10.1158/0008-5472.CAN-14-3098 (2015).
Wimberly, H. et al. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer immunology research 3, 326–332, https://doi.org/10.1158/2326-6066.CIR-14-0133 (2015).
Zhang, P., Su, D. M., Liang, M. & Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Molecular immunology 45, 1470–1476, https://doi.org/10.1016/j.molimm.2007.08.013 (2008).
Van Der Kraak, L. et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. Journal for immunotherapy of cancer 4, 65, https://doi.org/10.1186/s40425-016-0163-8 (2016).
Donnem, T. et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology 27, 225–232, https://doi.org/10.1093/annonc/mdv560 (2016).
Tokito, T. et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer 55, 7–14, https://doi.org/10.1016/j.ejca.2015.11.020 (2016).
Taube, J. M. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 3, e963413, https://doi.org/10.4161/21624011.2014.963413 (2014).
Dong, Z. Y., Wu, S. P., Liao, R. Q., Huang, S. M. & Wu, Y. L. Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 4251–4261, https://doi.org/10.1007/s13277-016-4812-9 (2016).
Wang, W. et al. PD-1 mRNA expression in peripheral blood cells and its modulation characteristics in cancer patients. Oncotarget, doi:https://doi.org/10.18632/oncotarget.15006 (2017).
Bernstein, M. B. et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer biotherapy & radiopharmaceuticals 29, 153–161, https://doi.org/10.1089/cbr.2013.1578 (2014).
van Meir, H. et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 6, e1267095, https://doi.org/10.1080/2162402X.2016.1267095 (2017).
Teng, F. et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Translational research: the journal of laboratory and clinical medicine 166, 721–732 e721, https://doi.org/10.1016/j.trsl.2015.06.019 (2015).
Stamell, E. F., Wolchok, J. D., Gnjatic, S., Lee, N. Y. & Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. International journal of radiation oncology, biology, physics 85, 293–295, https://doi.org/10.1016/j.ijrobp.2012.03.017 (2013).
Slovin, S. F. et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Annals of oncology: official journal of the European Society for Medical Oncology 24, 1813–1821, https://doi.org/10.1093/annonc/mdt107 (2013).
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A. & Formenti, S. C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer immunology research 1, 365–372, https://doi.org/10.1158/2326-6066.CIR-13-0115 (2013).
Bezjak, A. et al. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33, 2100–2105, https://doi.org/10.1200/JCO.2014.59.2360 (2015).
Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 2, 706–714, https://doi.org/10.1097/JTO.0b013e31812f3c1a (2007).
Gaule, P. et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA oncology, doi:https://doi.org/10.1001/jamaoncol.2016.3015 (2016).
Phillips, T. et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Applied immunohistochemistry & molecular morphology: AIMM 23, 541–549, https://doi.org/10.1097/PAI.0000000000000256 (2015).
Neuman, T. et al. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana’s Platform. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 11, 1863–1868, https://doi.org/10.1016/j.jtho.2016.08.146 (2016).
Reck, M. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 375, 1823–1833, https://doi.org/10.1056/NEJMoa1606774 (2016).
Donnem, T. et al. Stromal CD8+ T-cell Density-A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 21, 2635–2643, https://doi.org/10.1158/1078-0432.CCR-14-1905 (2015).
Al-Shibli, K. I. et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 14, 5220–5227, https://doi.org/10.1158/1078-0432.CCR-08-0133 (2008).
Acknowledgements
This study was supported by the Kasahara Cancer Research Fund and internal funding. This study was approved by the Ethics Committee of Kobe City Medical Center General Hospital. The authors would like to thank Keiko Sakuragawa and Ayako Okamoto for their administrative assistance and Masafumi Sugahara and Syuji Imoto for pathological analysis.
Author information
Authors and Affiliations
Contributions
D.F. wrote the initial draft of the manuscript, designed the study, and contributed to analysis and interpretation of data. K.U. and Y.S. contributed to statistical analysis and interpretation of data. K.U., Y.S., I.S., M.I., S.T., K.N., A.N., Y.K., K.O., Y.I., H.H., Y.T., M.K., and K.T. have contributed to data collection and interpretation, and critically reviewed the manuscript. The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujimoto, D., Uehara, K., Sato, Y. et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep 7, 11373 (2017). https://doi.org/10.1038/s41598-017-11949-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11949-9
This article is cited by
-
Impact of platinum-based chemotherapy on the tumor mutational burden and immune microenvironment in non-small cell lung cancer with postoperative recurrence
Clinical and Translational Oncology (2024)
-
Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers
Molecular Cancer (2023)
-
Impact of TMB/PD-L1 expression and pneumonitis on chemoradiation and durvalumab response in stage III NSCLC
Nature Communications (2023)
-
Influence of chemoradiation on the immune microenvironment of cervical cancer patients
Strahlentherapie und Onkologie (2023)
-
Analytical validation and initial clinical testing of quantitative microscopic evaluation for PD-L1 and HLA I expression on circulating tumor cells from patients with non-small cell lung cancer
Biomarker Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.