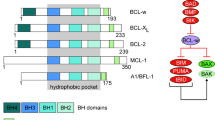
Abstract
The loss of vital cells within healthy tissues contributes to the development, progression and treatment outcomes of many human disorders, including neurological and infectious diseases as well as environmental and medical toxicities. Conversely, the abnormal survival and accumulation of damaged or superfluous cells drive prominent human pathologies such as cancers and autoimmune diseases. Apoptosis is an evolutionarily conserved cell death pathway that is responsible for the programmed culling of cells during normal eukaryotic development and maintenance of organismal homeostasis. This pathway is controlled by the BCL-2 family of proteins, which contains both pro-apoptotic and pro-survival members that balance the decision between cellular life and death. Recent insights into the dynamic interactions between BCL-2 family proteins and how they control apoptotic cell death in healthy and diseased cells have uncovered novel opportunities for therapeutic intervention. Importantly, the development of both positive and negative small-molecule modulators of apoptosis is now enabling researchers to translate the discoveries that have been made in the laboratory into clinical practice to positively impact human health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rathmell, J. C. & Thompson, C. B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109, S97–S107 (2002).
Sedger, L. M. et al. Extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia in FasL and TRAIL double-deficient mice. Blood 115, 3258–3268 (2010).
Lamhamedi-Cherradi, S. E., Zheng, S. J., Maguschak, K. A., Peschon, J. & Chen, Y. H. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat. Immunol. 4, 255–260 (2003).
Su, J. H., Deng, G. & Cotman, C. W. Bax protein expression is increased in Alzheimer’s brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J. Neuropathol. Exp. Neurol. 56, 86–93 (1997).
Lu, T. et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature 507, 448–454 (2014).
Paulino, A. C., Constine, L. S., Rubin, P. & Williams, J. P. Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin. Radiat. Oncol. 20, 12–20 (2010).
Nakaya, K. et al. Sensitivity to radiation-induced apoptosis and neuron loss declines rapidly in the postnatal mouse neocortex. Int. J. Radiat. Biol. 81, 545–554 (2005).
Lipshultz, S. E., Cochran, T. R., Franco, V. I. & Miller, T. L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 10, 697–710 (2013).
Honarpour, N., Gilbert, S. L., Lahn, B. T., Wang, X. & Herz, J. Apaf-1 deficiency and neural tube closure defects are found in fog mice. Proc. Natl Acad. Sci. USA 98, 9683–9687 (2001).
Ke, F. F. S. et al. Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX, BAK, and BOK. Cell 173, 1217–1230 (2018). This study highlights the importance of apoptosis in mammalian development but also shows that a viable mouse can be born in the absence of intrinsic apoptosis.
Knudson, C. M., Tung, K. S., Tourtellotte, W. G. & Brown, G. a & Korsmeyer, S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, 96–99 (1995).
Eischen, C. M., Roussel, M. F., Korsmeyer, S. J. & Cleveland, J. L. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol. Cell. Biol. 21, 7653–7662 (2001).
Luke, J. J., Van De Wetering, C. I. & Knudson, C. M. Lymphoma development in Bax transgenic mice is inhibited by Bcl-2 and associated with chromosomal instability. Cell Death Differ. 10, 740–748 (2003).
Los, M. et al. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 375, 81–83 (1995).
Miura, M., Zhu, H., Rotello, R., Hartwieg, E. A. & Yuan, J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75, 653–660 (1993).
Sabbatini, P., Han, J., Chiou, S. K., Nicholson, D. W. & White, E. Interleukin 1 beta converting enzyme-like proteases are essential for p53-mediated transcriptionally dependent apoptosis. Cell Growth Differ. 8, 643–653 (1997).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018). This is a comprehensive overview and comparison of cell death modalities.
Cotter, T. G. Apoptosis and cancer: the genesis of a research field therapy. Nat. Rev. Cancer 9, 501–507 (2009).
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183–192 (2002).
Maas, C. et al. Smac/DIABLO release from mitochondria and XIAP inhibition are essential to limit clonogenicity of Type I tumor cells after TRAIL receptor stimulation. Cell Death Differ. 17, 1613–1623 (2010).
Brunet, C. L. et al. Commitment to cell death measured by loss of clonogenicity is separable from the appearance of apoptotic markers. Cell Death Differ. 5, 107–115 (1998).
Marsden, V. S. et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 6–9 (2002).
Tait, S. W. G. & Green, D. R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 5, a008706 (2013).
Tait, S. W. G. et al. Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev. Cell 18, 802–813 (2010).
Ichim, G. et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 57, 860–872 (2015).
Liu, X. et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol. Cell 58, 1–13 (2015).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type i IFN production. Cell 159, 1549–1562 (2014).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018).
Riley, J. et al. Activated BAX/BAK enable mitochondrial inner membrane permeabilisation and mtDNA release during cell death. Preprint at https://www.biorxiv.org/content/early/2018/02/26/272104 (2018). References 29 and 30 describe the role of BAX/BAK pores in the transfer of mitochondrial DNA into the cytoplasm, where it can activate pro-inflammatory responses.
Vanpouille-Box, C., Demaria, S., Formenti, S. C. & Galluzzi, L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell 34, 361–378 (2018).
West, A. P. & Shadel, G. S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17, 363–375 (2017).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell. Biol. 15, 49–63 (2013).
Czabotar, P. E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 (2013). This study demonstrates that BAX is activated by a pro-apoptotic peptide to unify competing models of activation.
Kim, H. et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 8, 1348–1358 (2006).
Leshchiner, E. S., Braun, C. R., Bird, G. H. & Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proc. Natl Acad. Sci. USA 110, E986–E995 (2013).
Wei, M. et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060–2071 (2000).
Dai, H., Pang, Y.-P., Ramirez-Alvarado, M. & Kaufmann, S. H. Evaluation of the BH3-only protein Puma as a direct Bak activator. J. Biol. Chem. 289, 89–99 (2013).
Glab, J. A., Mbogo, G. W. & Puthalakath, H. BH3-only proteins in health and disease. Int. Rev. Cell Mol. Biol. 328, 163–196 (2017).
Sarosiek, K. A. et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell 51, 751–765 (2013).
Kuwana, T. et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525–535 (2005).
Uren, R. T. et al. Mitochondrial permeabilization relies on BH3 ligands engaging multiple prosurvival Bcl-2 relatives, not Bak. J. Cell Biol. 177, 277–287 (2007).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006).
Opferman, J. T. et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 570–574 (2003).
Sarosiek, K. A. et al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 31, 142–156 (2017). This paper provides a demonstration that apoptotic priming, which is dynamically and differently regulated across healthy tissues, is important for cellular sensitivity to damage or stress.
Moldoveanu, T. et al. BID-induced structural changes in BAK promote apoptosis. Nat. Struct. Mol. Biol. 20, 589–597 (2013).
O’Neill, K. L., Huang, K., Zhang, J., Chen, Y. & Luo, X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 30, 973–988 (2016).
Eriksson, D. & Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 31, 363–372 (2010).
Kaufmann, S. H. & Earnshaw, W. C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 256, 42–49 (2000).
Corazzari, M., Gagliardi, M., Fimia, G. M. & Piacentini, M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front. Oncol. 7, 78 (2017).
Sarosiek, K. A. et al. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc. Natl Acad. Sci. USA 107, 13069–13074 (2010).
Panduri, V., Weitzman, S. A., Chandel, N. S. & Kamp, D. W. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Cell. Mol. Physiol. 286, L1220–L1227 (2004).
Wong, K.-K., Engelman, J. A. & Cantley, L. C. Targeting the PI3K signaling pathway in cancer. Curr. Opin. Genet. Dev. 20, 87–90 (2010).
Sarosiek, K. A. K. et al. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B cell lymphomas. Blood 115, 570–580 (2010).
Wensveen, F. M. et al. Apoptosis threshold set by noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity 32, 754–765 (2010).
Murphy, D. J. et al. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell 14, 447–457 (2008).
Phesse, T. J. et al. Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo. Cell Death Differ. 44, 956–966 (2014).
Garrison, S. P. et al. Selection against PUMA gene expression in Myc-driven B cell lymphomagenesis. Mol. Cell. Biol. 28, 5391–5402 (2008).
Evan, G., Wyllie, A., Gilbert, C. & Littlewood, T. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992).
Soucie, E. L. et al. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol. Cell. Biol. 21, 4725–4736 (2001).
Farlie, P. G., Dringen, R., Rees, S. M., Kannourakis, G. & Bernard, O. bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc. Natl Acad. Sci. USA 92, 4397–4401 (1995).
Mercille, S. & Massie, B. Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotechnol. Bioeng. 44, 1140–1154 (1994).
Wiederschain, D., Kawai, H., Shilatifard, A. & Yuan, Z.-M. Multiple mixed lineage leukemia (MLL) fusion proteins suppress p53-mediated response to DNA damage. J. Biol. Chem. 280, 24315–24321 (2005).
de Polo, A. et al. AXL receptor signalling suppresses p53 in melanoma through stabilization of the MDMX-MDM2 complex. J. Mol. Cell. Biol. 9, 154–165 (2017).
Deng, J. et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 67, 11867–11875 (2007).
Hata, A. N. et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res. 74, 3146–3156 (2014).
Winter, P. S. et al. RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci. Signal. 7, 1–12 (2014).
Lei, K. & Davis, R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA 100, 2432–2437 (2003).
Putcha, G. V. et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38, 899–914 (2003).
Wong, W. W.-L. & Puthalakath, H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life 60, 390–397 (2008).
Ni Chonghaile, T. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011).
Sarosiek, K. A., Ni Chonghaile, T. & Letai, A. Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol. 23, 1–8 (2013).
Sarosiek, K. A. & Letai, A. Directly targeting the mitochondrial pathway of apoptosis for cancer therapy with BH3 mimetics: recent successes, current challenges and future promise. FEBS J. 283, 3523–3533 (2016).
Ryan, J. & Letai, A. BH3 profiling in whole cells by fluorimeter or FACS. Methods 61, 156–164 (2013).
Opferman, J. T. & Korsmeyer, S. J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4, 410–415 (2003).
Madden, S. D., Donovan, M. & Cotter, T. G. Key apoptosis regulating proteins are down-regulated during postnatal tissue development. Int. J. Dev. Biol. 51, 415–423 (2007).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Honarpour, N. et al. Adult Apaf-1-deficient mice exhibit male infertility. Dev. Biol. 218, 248–258 (2000).
Arakawa, S. et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 24, 1598–1608 (2017).
Garcia, I. et al. Bax deficiency prolongs cerebellar neurogenesis, accelerates medulloblastoma formation and paradoxically increases both malignancy and differentiation. Oncogene 32, 2304–2314 (2013).
Southwell, D. G. et al. Intrinsically determined cell death of developing cortical interneurons. Nature 491, 109–113 (2012).
Deckwerth, T. L. et al. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17, 401–411 (1996).
Merchant, T. E., Pollack, I. F. & Loeffler, J. S. Brain tumors across the age spectrum: biology, therapy, and late effects. Semin. Radiat. Oncol. 20, 58–66 (2010).
Crowther, A. J. et al. Tonic activation of Bax primes neural progenitors for rapid apoptosis through a mechanism preserved in medulloblastoma. J. Neurosci. 33, 18098–18108 (2013).
Lidsky, T. I. & Schneider, J. S. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126, 5–19 (2003).
Grandjean, P. & Landrigan, P. Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178 (2006).
Andropoulos, D. B. Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn. Ther. 43, 1–11 (2018).
Heine, V. M. & Rowitch, D. H. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11βHSD2-dependent mechanism. J. Clin. Invest. 119, 267–277 (2009).
Thornton, C. et al. Cell death in the developing brain after hypoxia-ischemia. Front. Cell. Neurosci. 11, 248 (2017).
Biddle, K. R., McCabe, A. & Bliss, L. S. Narrative skills following traumatic brain injury in children and adults. J. Commun. Disord. 29, 447–468 (1996).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995).
Mitchell, K. O. et al. Bax is a transcriptional target and mediator of c-Myc-induced apoptosis. Cancer Res. 60, 6318–6325 (2000).
Strasser, A., Harris, A. W. & Cory, S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67, 889–899 (1991).
Okamoto, T. et al. Enhanced stability of Mcl1, a prosurvival Bcl2 relative, blunts stress-induced apoptosis, causes male sterility, and promotes tumorigenesis. Proc. Natl Acad. Sci. USA 111, 261–266 (2014).
Shaha, C., Tripathi, R. & Prasad Mishra, D. Male germ cell apoptosis: regulation and biology. Phil. Trans. R. Soc. B 365, 1501–1515 (2010).
Rodriguez, I., Araki, K., Khatib, K., Martinou, J. C. & Vassalli, P. Mouse vaginal opening is an apoptosis-ependent process which can be prevented by the overexpression of Bcl2. Dev. Biol. 184, 115–121 (1997).
Rinkenberger, J. L., Horning, S., Klocke, B., Roth, K. & Korsmeyer, S. J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14, 23–27 (2000).
Escudero, S. et al. Dynamic regulation of long-chain fatty acid oxidation by a noncanonical interaction between the MCL-1 BH3 helix and VLCAD. Mol. Cell 69, 729–743 (2018).
Perciavalle, R. M. et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat. Cell Biol. 14, 575–583 (2012).
Pillay, J. et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116, 625–627 (2010).
Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147, 765–783 (2014).
Pilzecker, B. et al. DNA damage tolerance in hematopoietic stem and progenitor cells in mice. Proc. Natl Acad. Sci. USA 114, 201706508 (2017).
Gutierrez-Martinez, P. et al. Diminished apoptotic priming and ATM signalling confer a survival advantage onto aged haematopoietic stem cells in response to DNA damage. Nat. Cell Biol. 20, 413–421 (2018).
Mason, K. D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Vlahovic, G. et al. A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Invest. New Drugs 32, 976–984 (2014).
Opferman, J. T. & Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 25, 37–45 (2018).
Spetz, J., Presser, A. G. & Sarosiek, K. A. T cells and regulated cell death: kill or be killed. Int. Rev. Cell Mol. Biol. https://doi.org/10.1016/bs.ircmb.2018.07.004 (2018).
Zhao, D. Y., Jacobs, K. M., Hallahan, D. E. & Thotala, D. Silencing Egr1 attenuates radiation-induced apoptosis in normal tissues while killing cancer cells and delaying tumor growth. Mol. Cancer Ther. 14, 2343–2352 (2015).
Spetz, J., Moslehi, J. & Sarosiek, K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr. Treat. Options Cardiovasc. Med. 20, 31 (2018).
Schuler, F. et al. The BH3-only protein BIM contributes to late-stage involution in the mouse mammary gland. Cell Death Differ. 23, 41–51 (2016).
Bergmann, A. & Steller, H. Apoptosis, stem cells, and tissue regeneration. Sci. Signal. 3, re8 (2010).
Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 5, e996 (2014).
Bakhshi, A. et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around Jhon chromosome 14 and near a transcriptional unit on 18. Cell 41, 899–906 (1985).
Tang, H. L. et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 23, 2240–2252 (2012).
Reed, J. C. Bcl-2 on the brink of breakthroughs in cancer treatment. Cell Death Differ. 25, 3–6 (2018).
Whitfield, J. R., Beaulieu, M.-E. & Soucek, L. Strategies to inhibit Myc and their clinical applicability. Front. Cell Dev. Biol. 5, 10 (2017).
Hanahan, D. & Weinberg, R. The hallmarks of cancer. Cell 100, 57–70 (2000).
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348, 331–333 (1990).
Lopez, J. & Tait, S. W. G. Mitochondrial apoptosis: killing cancer using the enemy within. Br. J. Cancer 112, 957–962 (2015).
Birkinshaw, R. W. & Czabotar, P. E. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol. 72, 152–162 (2017).
Leverson, J. D. et al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor venetoclax. Cancer Discov. 7, 1376–1393 (2017).
Green, D. R. Bench to bedside a BH3 mimetic for killing cancer cells. Cell 165, 1560 (2016).
Miquel, C. et al. Role of bax mutations in apoptosis in colorectal cancers with microsatellite instability. Am. J. Clin. Pathol. 123, 562–570 (2005).
Gardai, S. J. et al. Phosphorylation of Bax ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 279, 21085–21095 (2004).
Kutuk, O. & Letai, A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr. Mol. Med. 8, 102–118 (2008).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30 (2017).
Huber, H. J., McKiernan, R. G. & Prehn, J. H. M. Harnessing system models of cell death signalling for cytotoxic chemotherapy: towards personalised medicine approaches? J. Mol. Med. 92, 227–237 (2014).
Mitchison, T. J. The proliferation rate paradox in antimitotic chemotherapy. Mol. Biol. Cell 23, 1–6 (2012). This paper provides a balanced discussion on the proliferation rate of cancer cells and their sensitivity to chemotherapy.
Sack, L. M. et al. Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns. Cell 173, 499–514 (2018).
Watanabe-Fukunaga, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999).
Strasser, A. et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl Acad. Sci. USA 88, 8661–8665 (1991).
Ina, K. et al. Resistance of Crohn’s disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J. Immunol. 163, 1081–1090 (1999).
Parandhaman, D. K. & Narayanan, S. Cell death paradigms in the pathogenesis of Mycobacterium tuberculosis infection. Front. Cell. Infect. Microbiol. 4, 31 (2014).
Zhou, X., Jiang, W., Liu, Z., Liu, S. & Liang, X. Virus infection and death receptor-mediated apoptosis. Viruses 9, 316 (2017).
Sly, L. M., Hingley-Wilson, S. M., Reiner, N. E. & McMaster, W. R. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170, 430–437 (2003).
Banga, S. et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc. Natl Acad. Sci. USA 104, 5121–5126 (2007).
Pauleau, A.-L. et al. Structure–function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 26, 7067–7080 (2007).
Desbien, A. L., Kappler, J. W. & Marrack, P. The Epstein–Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl Acad. Sci. USA 106, 5663–5668 (2009).
Flanagan, A. M. & Letai, A. BH3 domains define selective inhibitory interactions with BHRF-1 and KSHV BCL-2. Cell Death Differ. 15, 580–588 (2008).
Rowe, M. et al. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B cell-specific response that is delayed relative to NF-kappa B activation and to induction of cell surface markers. J. Virol. 68, 5602–5612 (1994).
Wang, S., Rowe, M. & Lundgren, E. Expression of the Epstein Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl-2 homologue Mcl-1 levels in B− cell lines. Cancer Res. 56, 4610–4613 (1996).
D’Souza, B., Rowe, M. & Walls, D. The bfl-1 gene is transcriptionally upregulated by the Epstein-Barr virus LMP1, and its expression promotes the survival of a Burkitt’s lymphoma cell line. J. Virol. 74, 6652–6658 (2000).
Ko, Y. H. Editorial: EBV and human cancer. Exp. Mol. Med. 47, e130–e133 (2015).
Collison, J. Targeting Bcl-2 prevents nephritis in mice. Nat. Rev. Rheumatol. 12, 376–376 (2016).
Wang, L. C. et al. ABT-199, a potent and selective BCL-2 inhibitor, prevents lupus nephritis in the spontaneous NZB/W F1 mouse model by depleting selective lymphocyte populations while sparing platelets [abstract 858]. Arthritis Rheumatol. 66, S379–S380 (2014).
Minocha, M., Zeng, J., Medema, J. K. & Othman, A. A. Pharmacokinetics of the B-cell lymphoma 2 (Bcl-2) inhibitor venetoclax in female subjects with systemic lupus erythematosus. Clin. Pharmacokinet. 2, 1–14 (2018).
Speir, M. et al. Eliminating Legionella by inhibiting BCL-XL to induce macrophage apoptosis. Nat. Microbiol. 1, 15034 (2016).
Okouchi, M., Ekshyyan, O., Maracine, M. & Aw, T. Y. Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal. 9, 1059–1096 (2007).
Radi, E., Formichi, P., Battisti, C. & Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 42, S125–S152 (2014).
Wright, K. M. & Deshmukh, M. Restricting apoptosis for postmitotic cell survival and its relevance to cancer. Cell Cycle 5, 1616–1620 (2006).
Kole, aJ., Annis, R. P. & Deshmukh, M. Mature neurons: equipped for survival. Cell Death Dis. 4, e689 (2013).
Polster, B. M., Robertson, C. L., Bucci, C. J., Suzuki, M. & Fiskum, G. Postnatal brain development and neural cell differentiation modulate mitochondrial Bax and BH3 peptide-induced cytochrome c release. Cell Death Differ. 10, 365–370 (2003).
Rohn, T. T. The role of caspases in Alzheimer’s disease: potential novel therapeutic opportunities. Apoptosis 15, 1403–1409 (2010).
Siegel, S. J., Bieschke, J., Powers, E. T. & Kelly, J. W. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46, 1503–1510 (2007).
Jembrek, M. J., Hof, P. R. & Šimic, G. Ceramides in Alzheimer’s disease: key mediators of neuronal apoptosis induced by oxidative stress and Aβ accumulation. Oxid. Med. Cell. Longev. 2015, 1–17 (2015).
Paradis, E., Douillard, H., Koutroumanis, M., Goodyer, C. & LeBlanc, A. Amyloid beta peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons. J. Neurosci. 16, 7533–7539 (1996).
Crews, L., Rockenstein, E. & Masliah, E. APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct. Funct. 214, 111–126 (2010).
Kitamura, Y. et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 780, 260–269 (1998).
Rohn, T. T. et al. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J. Neurosci. 28, 3051–3059 (2008).
Blesa, J. & Przedborski, S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front. Neuroanat. 8, 155 (2014).
Wood-Kaczmar, A. et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLOS ONE 3, e2455 (2008).
Berger, A. K. et al. Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum. Mol. Genet. 18, 4317–4328 (2009).
Wu, D. et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc. Natl Acad. Sci. USA 104, 8161–8166 (2007).
Ghavami, S. et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 112, 24–49 (2014).
Reyes, N. A. et al. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. Control 120, 3673–3679 (2010). This study demonstrates the functional impact of apoptosis prevention in a mouse model of ALS.
Saudou, F., Finkbeiner, S., Devys, D. & Greenberg, M. E. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998).
Hickey, M. A. & Chesselet, M.-F. Apoptosis in Huntington’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 255–265 (2003).
Sagot, Y. et al. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J. Neurosci. 15, 7727–7733 (1995).
Broughton, B. R. S., Reutens, D. C. & Sobey, C. G. Apoptotic mechanisms after cerebral ischemia. Stroke 40, e331–e339 (2009).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Plesnila, N. et al. Function of BID — a molecule of the bcl-2 family — in ischemic cell death in the brain. Eur. Surg. Res. 34, 37–41 (2002).
Gill, R. et al. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J. Cereb. Blood Flow Metab. 22, 420–430 (2002).
Gibson, M. E. et al. BAX contributes to apoptotic-like death following neonatal hypoxia-ischemia: evidence for distinct apoptosis pathways. Mol. Med. 7, 644–655 (2001).
Zhang, X., Chen, Y., Jenkins, L. W., Kochanek, P. M. & Clark, R. S. B. Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit. Care 9, 66–75 (2005).
Raghupathi, R., Graham, D. I. & McIntosh, T. K. Apoptosis after traumatic brain injury. J. Neurotrauma 17, 927–938 (2000).
Kohda, K. et al. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut 44, 456–462 (1999).
Yamasaki, E. et al. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J. Biol. Chem. 281, 11250–11259 (2006).
Matsumoto, A. et al. Helicobacter pylori VacA reduces the cellular expression of STAT3 and pro-survival Bcl-2 family proteins, Bcl-2 and Bcl-XL, leading to apoptosis in gastric epithelial cells. Dig. Dis. Sci. 56, 999–1006 (2011).
Strack, P. R. et al. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc. Natl Acad. Sci. USA 93, 9571–9576 (1996).
Roggero, R. et al. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 75, 7637–7650 (2001).
Ullrich, C. K., Groopman, J. E. & Ganju, R. K. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood 96, 1438–1442 (2000).
Boudet, F., Lecoeur, H. & Gougeon, M. L. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up- regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J. Immunol. 156, 2282–2293 (1996).
Cambier, C. J., Falkow, S. & Ramakrishnan, L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 159, 1497–1509 (2014).
Schulz, J. B. et al. Extended therapeutic window for caspase inhibition and synergy with MK-801 in the treatment of cerebral histotoxic hypoxia. Cell Death Differ. 5, 847–857 (1998).
Venero, J. L., Burguillos, M. A. & Joseph, B. Caspases playing in the field of neuroinflammation: old and new players. Dev. Neurosci. 35, 88–101 (2013).
Clark, R. S. et al. boc-Aspartyl(OMe)-fluoromethylketone attenuates mitochondrial release of cytochrome c and delays brain tissue loss after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 27, 316–326 (2007).
Cheng, Y. et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J. Clin. Invest. 101, 1992–1999 (1998).
Han, B. H. et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J. Biol. Chem. 277, 30128–30136 (2002).
Niu, X. et al. A small-molecule inhibitor of Bax and Bak oligomerization prevents genotoxic cell death and promotes neuroprotection. Cell Chem. Biol. 24, 493–506 (2017). This paper is one of the first reports of small molecules able to bind and inactivate BAX and BAK for potential neuroprotection.
Hetz, C. et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 280, 42960–42970 (2005).
Pan, R. et al. Selective BCL-2 inhibition by ABT-199 causes on target cell death in acute myeloid leukemia. Cancer Discov. 4, 362–375 (2013).
Juin, P., Geneste, O., Gautier, F., Depil, S. & Campone, M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat. Rev. Cancer 13, 455–465 (2013).
Leverson, J. D. et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl Med. 7, 279ra40 (2015).
Pécot, J. et al. Tight sequestration of BH3 proteins by BCL-xL at subcellular membranes contributes to apoptotic resistance. Cell Rep. 17, 3347–3358 (2016).
Konopleva, M. et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10, 375–388 (2006).
Goldsmith, K. C. et al. Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer Res. 72, 2565–2577 (2012).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2015). This study provides a demonstration of the clinical utility of BH3 mimetics for the treatment of cancer.
Schenk, R. L., Strasser, A. & Dewson, G. BCL-2: long and winding path from discovery to therapeutic target. Biochem. Biophys. Res. Commun. 482, 459–469 (2017).
Letai, A. S63845, an MCL-1 selective BH3 mimetic: another arrow in our quiver. Cancer Cell 30, 834–835 (2016).
Vogler, M. et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 117, 7145–7154 (2011).
Al-harbi, S. et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood 118, 3579–3590 (2011).
Slee, Ea, Keogh, Sa & Martin, S. J. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7, 556–565 (2000).
Suzuki, Y. et al. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8, 613–621 (2001).
Srinivasula, S. M. et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410, 112–116 (2001).
Toshiyuki, M. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Zong, W. X., Edelstein, L. C., Chen, C., Bash, J. & Gélinas, C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 13, 382–387 (1999).
Grossmann, M. et al. The anti-apoptotic activities of Rel and RelA required during B cell maturation involve the regulation of Bcl-2 expression. EMBO J. 19, 6351–6360 (2000).
Kale, J., Osterlund, E. J. & Andrews, D. W. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 25, 65–80 (2017).
Chi, X., Kale, J., Leber, B. & Andrews, D. W. Regulating cell death at, on, and in membranes. Biochim. Biophys. Acta 1843, 2100–2113 (2014).
Kalkavan, H. & Green, D. R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25, 1–10 (2017).
Popgeorgiev, N., Jabbour, L. & Gillet, G. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front. Cell Dev. Biol. 6, 1–11 (2018).
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Brahmbhatt, H., Uehling, D., Al-awar, R., Leber, B. & Andrews, D. Small molecules reveal an alternative mechanism of Bax activation. Biochem. J. 473, 1073–1083 (2016).
Chonghaile, T. N. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011).
Ryan, J., Montero, J., Rocco, J. & Letai, A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol. Chem. 397, 671–678 (2016).
Davids, M. S. et al. Mitochondrial apoptotic priming is associated with clinical response to the Bcl-2 antagonist ABT-199 in chronic lymphocytic leukemia. Blood 124, 1940 (2014).
Vo, T.-T. et al. Relative mitochondrial priming of malignant myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012).
Del, V. et al. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Leukemia 111, 2300–2309 (2008).
Ni Chonghaile, T. et al. Maturation stage of T cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 4, 1074–1087 (2014).
Deng, J. et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12, 171–185 (2007).
Montero, J. et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989 (2015).
Montero, J. et al. Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL-2 and sensitive to venetoclax. Cancer Discov. 7, 156–164 (2016).
Acknowledgements
The authors acknowledge the many researchers who contributed to their understanding of apoptosis and apologize that they could not cite all the relevant research because of space restrictions. The authors thank B. Croker, G. Joshi, A. Presser, J. Spetz, K. Webster and T. Gershon for critical feedback and helpful discussions. The authors gratefully acknowledge funding from the Alex’s Lemonade Stand Foundation for Childhood Cancers Young Investigator Award (K.S.), Andrew McDonough B+ Foundation Childhood Cancer Research Grant (K.S.), Harvard T.H. Chan School of Public Health Dean’s Fund for Scientific Advancement (K.S.), Making Headway Foundation St Baldrick’s Research Grant (K.S.) and NIH/NCI grant R00CA188679 (K.S.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Death receptor
-
A subgroup of the tumour necrosis factor receptor (TNFR) superfamily that can activate the extrinsic apoptosis pathway via a conserved cytoplasmic signalling platform called the death domain. Prominent members of this family include FAS (also known as Apo1 and CD95), TNFR1 and TRAIL.
- Cytochrome c
-
An essential component of the electron transport chain within mitochondria, where it carries electrons. When released from mitochondria as a result of BCL-2-associated X protein (BAX) and/or BCL 2 antagonist/killer (BAK) activation, cytochrome c has a prominent role in controlling the commitment to apoptosis — it binds to apoptotic protease-activating factor 1 (APAF1) to form an apoptosome, which activates caspases.
- Apoptosome
-
A quaternary protein complex composed of cytoplasmic cytochrome c, apoptotic protease-activating factor 1 (APAF1) and dATP that recruits and activates the normally inactive pro-caspase 9, which then activates effector caspases to prepare the cell for phagocytosis.
- Second mitochondria-derived activator of caspases
-
(SMAC). Also known as DIABLO. A protein that is released from mitochondria during mitochondrial outer membrane permeabilization to bind and inactivate X-linked inhibitor of apoptosis protein (XIAP) to promote caspase activation.
- X-linked inhibitor of apoptosis protein
-
(XIAP). A member of the inhibitor of apoptosis family of proteins that can prevent caspase activation. XIAP binds and inactivates caspases 3, 7 and 9 via its baculovirus IAP repeat BIR2 and BIR3 domains.
- cGAS–STING
-
Cyclic GMP–AMP synthase (cGAS) is a DNA-sensing molecule that activates innate immune responses through production of the second messenger, cyclic GMP–AMP (cGAMP), which then binds to and activates the adaptor protein stimulator of interferon genes (STING). Importantly, cGAS is activated by double-stranded DNA that can be foreign or self.
- Damage-associated molecular pattern
-
(DAMP). A signal that initiates and perpetuates immune activation in response to tissue damage, trauma or ischaemia regardless of whether pathogens are present at the site of injury.
- Endoplasmic reticulum stress
-
(ER stress). A response of the ER to aberrations of protein folding (and other stresses), which is aimed at clearing unfolded proteins and restoring ER homeostasis. In cases when this cannot be accomplished, cellular functions degenerate and often result in apoptosis.
- Activation-induced cell death
-
A programmed cell death process initiated in immune cells (especially T cells) by repeated stimulation of their T cell receptors that helps to maintain peripheral immune tolerance.
- Ischaemia–reperfusion injury
-
A type of tissue damage resulting from initial ischaemia or hypoxia, followed by re-oxygenation, as seen in myocardial infarction, ischaemic stroke and other traumas.
- Sertoli cells
-
Elongated cells of the seminiferous tubules within the testis to which spermatids attach during spermatogenesis for support and nourishment.
- Double-negative T cells
-
(DNTCs). T cells expressing the T cell receptor but lacking CD4, CD8 or natural killer (NK) cell markers.
- Cell-cycle checkpoint violation
-
The failure of a cell to stop at specific checkpoints in the cell cycle when it would normally examine internal and external cues to determine whether to advance with cell division.
- Amyloid-β
-
(Aβ). A peptide produced through the proteolytic cleavage of a transmembrane protein, amyloid precursor protein (APP), by β-secretases and γ-secretases. Accumulation of Aβ in the brain is thought to be an early event in the pathogenesis of Alzheimer disease.
- Tau
-
The major microtubule-associated protein (MAP) of a normal postmitotic and mature neuron. Tau has six molecular isoforms that are generated by alternative splicing and is thought to promote the assembly of tubulin into microtubules. In Alzheimer disease and other tauopathies, tau is hyperphosphorylated and aggregates into neurofibrillary tangles to impair neuronal function.
- Neurofibrillary tangles
-
(NFTs). Aggregates of hyperphosphorylated tau proteins within neurons that cause dysfunction.
- 4-Hydroxynonenal
-
(4-HNE). A product of lipid peroxidation that can induce apoptosis.
- Ceramide
-
A lipid that acts as a second messenger in activating apoptosis within the sphingomyelin pathway, which is initiated by the hydrolysis of the plasma membrane phospholipid sphingomyelin.
- Presenilin-1
-
The catalytic subunit of γ-secretase, which is a protease that cleaves a variety of type 1 transmembrane proteins, most notably amyloid precursor protein. Mutations in the PSEN1 gene encoding presenilin 1 are the most common cause of familial Alzheimer disease.
- Chorea
-
A movement disorder that causes involuntary, unpredictable body movements.
- TUNEL
-
(Terminal deoxynucleotidyl transferase dUTP nick end labelling). An assay that detects fragmented DNA, which is one of the hallmarks of apoptotic cell death.
- Penumbra
-
In medicine, the area surrounding the focal point of an ischaemic event.
- Excitotoxicity
-
A process whereby cells in the nervous system are killed by excessive neurotransmitter stimulation.
Rights and permissions
About this article
Cite this article
Singh, R., Letai, A. & Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20, 175–193 (2019). https://doi.org/10.1038/s41580-018-0089-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-018-0089-8
This article is cited by
-
Crosstalk between metabolism and cell death in tumorigenesis
Molecular Cancer (2024)
-
Modulation of apoptosis and Inflammasome activation in chondrocytes: co-regulatory role of Chlorogenic acid
Cell Communication and Signaling (2024)
-
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
-
Multipronged regulation of autophagy and apoptosis: emerging role of TRIM proteins
Cellular & Molecular Biology Letters (2024)
-
A matter of new life and cell death: programmed cell death in the mammalian ovary
Journal of Biomedical Science (2024)