Abstract
Significant progress in the development of new immunotherapies has led to successful clinical trials for malignant melanoma and non-small cell lung cancer; however, for the majority of solid tumours of the gastrointestinal tract, little or no progress has been seen. The efficacy of immunotherapies is limited by the complexities of a diverse set of immune cells, and interactions between the tumour cells and all other cells in the local microenvironment of solid tumours. A large fraction of immune cells present in and around solid tumours derive from the innate arm of the immune system and using these cells against tumours offers an alternative immunotherapeutic option, especially as current strategies largely harness the adaptive arm of the immune system. This option is currently being investigated and attempts at using the innate immune system for gastrointestinal cancers are showing initial results. Several important factors, including cytokines, chemotherapeutics and the microbiome, influence the plasticity and functionality of innate (myeloid) cells in the microenvironment, and this complexity of regulation has limited translation into successful trials so far. In this review, current concepts of the immunobiology of the innate arm in the tumour microenvironment are presented in the context of clinical translation.
Similar content being viewed by others
Introduction
The local microenvironment of solid tumours is a complex system comprising cells of the immune system, fibroblasts, endothelial cells and many other cell types.1,2,3 Immune cells have different roles in the microenvironment, including pro-tumorigenic4 and anti-tumorigenic5 functions. Conceptually, the immune system can be divided into two major parts: the innate arm, which consists of an older evolutionary defence strategy, and the adaptive/acquired arm, which creates adaptive immunological memory. Although both arms of the immune system can be distinguished conceptually, they are functionally interlocking and thus heavily influence each other.6
One of the hallmarks of cancer is chronic inflammation,7 which fuels and sustains disease progression and neoplastic transformation;8 for colorectal cancer (CRC), this is most obviously evidenced for inflammatory bowel disease (IBD), which carries a significant risk of malignant transformation.9 Different sources of this inflammatory process have been identified, including persistent infections and sterile inflammation; for both of these sources, cells of the innate immune system can be the primary effector type. Although the extent of the individual contribution of these various innate cells to the primary inflammatory response is not precisely known, it is clear that dynamic changes in the microenvironment follow a specific pathway that is exploited by the tumour. The tumour-promoting pathway begins with continuous inflammatory signals provided by the tumour itself or via the host’s own immune system to eradicate the tumour cells. Inflammatory signals can consist of apoptotic cells, damage-associated molecular patterns, free DNA molecules, heat shock proteins and Toll-like receptors (TLRs)/ligands or cytokines, which may lead to the futile activation of immune cells.8,10,11,12 Subsequent chemokine production leads to an influx of more immune cells that can drive further activation or inactivation of immunological processes and can end up fuelling tumour growth and dissemination.
The main components of the innate immune system are physical epithelial barriers, phagocytic leukocytes (such as granulocytes and macrophages), dendritic cells, natural killer (NK)/innate lymphoid cells and circulating plasma proteins. This arm of the immune system is present in all tissues; however, its role in immunotherapy is poorly understood.7,13 Our understanding of the innate arm of the immune system and its complexities has been limited by the inherent fundamental functional differences between the immune system in animal models and in humans.14,15,16,17 Another factor contributing to our limited understanding concerns difficulties in identifying innate cell subsets in the local microenvironment through unambiguous surface markers reflecting functional states of cells; e.g., NK cells were long thought to be an influential factor in CRC and breast cancer, but analyses showed an unexpected absence of these cells from these tumours,18,19,20,21 despite the presence of chemokines and adhesion molecules. The different origins of myeloid cells22 and specific differentiation programmes for myeloid subtypes23 add another layer of complexity in regulation.
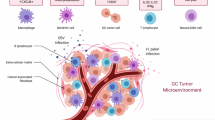
Looking into the composition of immune cells in solid tumours, myeloid cells can form a significant proportion of cells in the microenvironment, outnumbering lymphocytes and occasionally even the tumour cells themselves.24 Furthermore, fibroblasts and other mesenchymal cell types form an important component of the microenvironment,25 influencing hypoxia, migration of immune cells and the metastatic behaviour of tumour cells.26 This heterogeneity of the immune cell phenotypes present in the microenvironment across different cancer entities and metastatic sites (Fig. 1)27 is just one hurdle to overcome for successful immunotherapy; specific cellular distribution patterns (e.g., the exclusion or the dense infiltration of T cells in immune-excluded tumours), functional plasticity and organ-specific functions form a complex set-up that is further complicated by the influence of the tumour cells, all of which pose a challenge to therapeutic approaches. The complex interplay between the innate and adaptive arms of the immune system is, of course, also of relevance for therapeutic effects.
Overview of the composition of the immunological microenvironments in different lesions (primary vs. lung and liver metastases) of colorectal cancer (CRC). The pie charts provide examples of immune cell composition within the local microenvironment (data from Halama et al.27) to highlight organ-specific heterogeneity. The lower panel provides an overview of the key immune cells that are present in the immunological microenvironment
In contrast to the belief that the local immunological microenvironment of solid tumours is a chaotic and dysregulated site, we propose that it is a site with a specific pro-tumoural regulation. This review will discuss the immunobiology of the innate arm of the immune system in the microenvironment of CRC and the therapeutic potential of innate immune cells (with the exception of dendritic cells, see refs. 28,29,30) for immunotherapy.
The local microenvironment in CRC
Many publications have reported on the frequencies of immune cell subpopulations in different solid tumours and an association between immune cell density and clinical course has been shown by different groups for CRC.7,31,32,33,34,35,36 For many solid tumours, a high density of infiltrating T-effector cells is associated with a good prognosis and conversely a high density of myeloid cells is associated with a poor prognosis. Interestingly, the subpopulations relevant to tumour response and progression can vary between different cancers.37 Fridman38 proposed the concept of an ‘immune contexture’, which suggests that different compositions of immune cells and signalling molecules have specific roles in each cancer entity.
In CRC, the adaptive arm specifically has been shown not only to learn to recognise tumour cells but also to contribute greatly to the course of the disease. The presence of effector T cells in the local microenvironment is typically regarded as a sign of inflammation, whereas the presence of regulatory T cells is regarded as a sign of immunosuppression. High effector T-cell density is associated with a clear prognostic advantage across several different cancers; in CRC, the presence of effector T cells is linked to a good prognosis for both the primary tumour and in metastatic settings.39,40,41,42,43,44 The role of FOXP3 + regulatory T cells, however, is debated.45 Normally, regulatory T cells are regarded as immunosuppressive, abrogating an effective immune response against the tumour; however, in CRC, higher densities of these regulatory T cells are associated with a better prognosis, opposing the negative association of FOXP3– T lymphocytes in other cancer types. In metastatic liver lesions of CRC, the composition of the local microenvironment is mainly driven by chemokine gradients and cytokines, with only low numbers of NK cells or regulatory FOXP3 + T cells present.46 A small subgroup of patients with CRC have microsatellite-instable (MSI) tumours and show a massively increased presence of infiltrating adaptive immune cells (i.e., lymphocytes), with numbers more than twice as high as the average density in microsatellite-stable (MSS) CRC.47,48 In patients with MSI tumours, faulty DNA repair proteins give rise to more immunologically relevant mutations and produce a better control of the tumour through the immune system, which correlates with a better prognosis in these patients. Whereas MSI tumours respond well to immunotherapy, MSS CRC does not respond positively.
The role for B cells in the microenvironment is highly controversial in CRC, with data from quantification and localisation studies showing no clear significant association with clinical course in multivariable analysis.49 Future analyses should address the interplay between B cells and other innate immune cells in CRC.50 The presence of B cells and T cells together, as occurs in tertiary lymphoid structures, has been confirmed and analysed in CRC. In short, the presence of these tertiary lymphoid structures indicates a more favourable prognosis, owing to the increased infiltration of immune cells. There is also data, however, that associates tertiary lymphoid formation with BRAF mutation.51,52,53,54
Current immunotherapeutic approaches for CRC
Despite the multiple avenues that have been investigated to achieve tumour control, immunotherapy for CRC has so far largely failed to show clinically meaningful effects. Classic vaccination strategies have not shown significant effects; it remains to be seen whether more personalised approaches (e.g., mutanome vaccines based on sequencing efforts) will lead to effective vaccinations.55,56,57,58 Chimeric antigen receptor T-cell approaches have shown some positive effects; however, these were limited by severe toxicity or by efficacy limited to a specific mutation.59,60 A small subgroup of patients with mismatch-repair deficient (MMRd)/MSI CRC have shown good responses to checkpoint inhibition (e.g., via anti-PD-1, anti-PD-L1 or anti-CTLA-4: all three targets are present in the microenvironment)61 and this has led to the approval of anti-PD-1 antibodies for MSI CRC. This responsiveness to checkpoint inhibition most likely stems from the strong presence of T cells within the microenvironment in this subtype of CRC, as similar successes have not yet been reported for MSS CRC using checkpoint inhibition on its own.62,63,64,65,66 Systematic analyses of the mutational burden in MSS CRC has identified a subgroup of patients with high mutational burden but without MSI67,68; whether these patients would benefit from a systemic therapy with checkpoint inhibition remains unclear. Interestingly, a combined approach using chemotherapy (FOLFOX plus bevacizumab, NCT01633970) and anti-PD-L1 has shown some clinical effects in patients with (MSS) CRC.69
Determining why checkpoint inhibition does not work in patients with MSS CRC is a key question for immunotherapy. Resistance mechanisms in solid tumours are currently being systematically analysed; these mechanisms include induction of T-cell anergy via metabolic deprivation, inhibition of effector T-cell migration into the tumour tissue, T-cell inactivation via specific receptor–ligand interactions and barrier functions of the stroma, among others.70,71 Recent data suggest that some resistance mechanisms might be mediated by macrophages.72 In a broader approach, chemotherapy was combined with immunomodulation in the GOLFIG trials, in which a combination of gemcitabine, oxaliplatin, folinic acid, fluorouracil, interleukin (IL)-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) was administered.73,74 The initial data looked suggestive of enhanced efficacy; however, this approach was not continued due to recruitment problems and a modified protocol is being investigated (FOLFOXIGIL trial, NCT03222089). Broader still, histone deacetylase inhibitors have shown efficacy against lung cancer and other cancer entities, by inducing the reversal of T-cell exhaustion, among other means75; however, the effect of histone deacetylase inhibitors on macrophages and other immune cells in CRC is unclear.76
Other modulators of the immune microenvironment
Although the role of chronic inflammation as a driver for tumorigenesis is widely accepted (as mentioned above, chronic inflammation in IBD is associated with a higher risk of CRC), the role of inflammation and the immune system in non-IBD-mediated tumorigenesis is still unclear, especially as the role of non-steroidal anti-inflammatory drugs such as aspirin is still debated.77 Clinically, lower incidence rates of CRC and increased survival are associated with continual aspirin intake,78,79,80 but the molecular basis for this observation is not entirely clear81; however, the mutational status of the PI3KCA gene in tumour cells has been identified as one possible factor for the impact of aspirin.82,83,84 From the immunological standpoint, it is also not so clear. Although associations between the composition of the immunological microenvironment and aspirin intake have been observed,85 aspirin’s precise immunological mode of action remains unknown. More globally, we need to better understand the mechanisms of early carcinogenesis and the influence of adaptive and innate immunity at this stage, as well as the effect of other modulators of the immune system. For example, the level of vitamin D reportedly shows an association with the occurrence of CRC86 and clearly influences the composition of the local immunological tumour microenvironment87—higher plasma levels of vitamin D are associated with fewer tumours with higher T-cell infiltration. Precisely, how vitamin D influences monocytic cells in vivo remains unclear but differential modulation of the molecular response of monocytes, macrophages and dendritic cells to innate immune stimulation has been observed.88
Along the same lines, fatty acids have a profound role in modulating the local tumour microenvironment and the innate arm of the immune system.89 The association of a high intake of fibre with the suppression of inflammation is just one example of how nutrition can alter the local microenvironment in CRC90,91 and brings together the complexity of the areas of immunology and the microbiome.92,93 The influence of the microbiome on the innate immune system in CRC will be discussed below.
Immunotherapy for CRC: targeting innate immune cells
The composition and density of myeloid and non-myeloid immune cells in the CRC tumour microenvironment is surprisingly stable over time.94 Nevertheless, the plasticity of human myeloid cells and their lack of high-precision markers make it difficult to quantify, annotate and functionally characterise these cells. Their localisation and density together form an intrinsic network that reflects the activation and functionality of these cell populations, and requires sophisticated detection and quantification algorithms.95,96 Below we describe the key cells involved in innate immunity and current strategies to target them as a therapeutic approach to CRC.
Macrophages
Macrophages are among the most abundant cells within the CRC microenvironment and, together with myeloid-derived suppressor cells (MDSCs), they perform a diverse set of roles that includes skewing and suppressing adaptive immunity, orchestration of tissue repair and damage regulation, promotion of immunosuppression, modulation of the response to immunogenic cell death (‘adjuvanticity’), effector functionality against tumour cells and the mediation of abscopal effects.97 Macrophage plasticity is an important feature and the ability of different interventions (e.g., chemotherapy, radiation, etc.) to induce a rapid change in their function can be characterised, e.g., by changing from an immunosuppressive type II (M2) macrophage to an anti-tumour type I (M1) phenotype (Fig. 2). The net anti-tumoural effect can vary greatly (Table 1), although nearly all forms of intervention lead to modulation of macrophages in the tumour microenvironment. The factors that mediate this plasticity are highly diverse: cytokine and chemokine signals (IL-1, IL-4, IL-13, C–C motif chemokine ligand 5 (CCL5), CCL2, GM-CSF, CXCL12, IL-10, etc.) through to inflammation signals (e.g., prostaglandins, TLRs and ligands, complement system components), drugs (e.g., bisphosphonates) to metabolic and endocrine signals (e.g., fatty acids, lactate or vitamin D) and all forms of tissue stress (e.g., hypoxia, radiation) can modulate and alter macrophage functionality and consequently influence the neighbouring tumour.24,97,98,99,100,101 This influence can be both positive and negative, in an effect that is typically referred to as the ‘Yin–Yang’ of myeloid cells, whereby anti-tumoural effects as well as resistance to an intervention (e.g., chemotherapy with fluorouracil or bevacizumab) is mediated by the same cell type.24,102,103,104,105 Not only for CRC but also for all other cancer types, the functionality defining signals and signal combinations for macrophage modulation are starting to emerge, and with them the opportunity to design interventions.107 However, the precise role(s) of the presence of macrophages with different phenotypes in CRC is still being investigated and so far no clear-cut picture emerges,106 especially with respect to the mutational status of the tumour.108,109,110
Macrophage cell plasticity also translates to functional plasticity. Functionally relevant signals from within the microenvironment can influence whether macrophages adopt an anti-tumour type I (M1) phenotype or an immunosuppressive type II (M2) phenotype, or any intermediary complex phenotype. Different combinations of these signals can further dynamically affect macrophage differentiation and functionality
Different routes to target macrophages are being investigated in clinical trials (Table 1), ranging from macrophage depletion to macrophage repolarisation. As the name implies, depletion involves the destruction of macrophages in the tumour microenvironment, whereas the process of repolarisation tries to modulate the functional activity of the macrophages towards an anti-tumoural phenotype (i.e., cells that produce reactive oxygen species and interferons, or that phagocytose tumour cells). Strategies for abrogating macrophage recruitment to the specific organ or tumour tissue include the inhibition of chemokines and cytokines such as GM-CSF, vascular endothelial growth factor and CSF1, and modulation of pleiotropic cytokines such as macrophage migration inhibitory factor. Complement factor 5a also seems to have a role in the recruitment of myeloid populations into the tissue and into the tumour microenvironment. CCL2 is an example of a translational intervention aimed at modulating macrophage recruitment and data from a pancreatic ductal adenocarcinoma clinical trial combining CCL2 inhibition with chemotherapy are promising.110 Furthermore, CSF1–CSF-1R signalling is an important axis for the recruitment and generation of macrophage populations and extensive data from multiple groups have identified this signalling cascade as a central regulator of myeloid cell plasticity.24,111 Tumour responses were observed during clinical trials of a fully human CSF-1R antibody in patients with a rare diffuse-type giant-cell tumour;112 however, data from clinical trials in patients with malignant solid tumours show clear side effects and limited efficacy.111
Macrophage repolarisation therapy targeting the CCL5–CCR5 axis has been described in preclinical and clinical analyses for metastatic CRC.46,114,115,116 The chemokine effects of CCL5 on the migration of myeloid cells seems to have a minor role in this efficacy; rather, macrophage polarisation, with immediate effects on the production of interferon and reactive oxygen species, mediates these anti-tumoural effects and combination trials with checkpoint inhibitors are currently underway (NCT03631407 and NCT03274804). IL-1 inhibition has also shown encouraging effects in the clinic in patients with CRC. IL-1 inhibition has shown efficacy as a monotherapy (Table 1) as well as in combination with chemotherapy, and preclinical data suggest a myeloid-derived, IL-1-dependent tumour-promoting mechanism.116
Another approach targeting CRC is the use of TLR agonist and antagonist therapies. TLRs form a central regulatory unit in the defence against infectious agents and shape the behaviour or phenotype of CRC tumour cells.117 Two ongoing trials are currently evaluating the role of TLR agonists alone or in combination with chemotherapy. The role of vitamin D (or, more specifically, the modified vitamin D-binding protein macrophage activator EF-022) in macrophage activation is also being evaluated in clinical trials, thus potentially extending the beneficial effects of vitamin D beyond the adaptive arm of the immune system.118 In addition, another new avenue in the modulation of innate immune cells involves the combination therapy of atezolizumab (anti-PD-L1) with cobimetinib (MEK inhibition); it is assumed that synergistic myeloid cell modulation and parallel lymphocyte activation are induced, the precise mechanism of action in humans is not yet fully elucidated119 and clinical trial data has shown no effects in larger cohorts (IMblaze370 study120).
In contrast to these newer developments, two types of drug that have macrophage modulatory properties and a long history in medicine are bisphosphonates and trabectedin. Bisphosphonates have cytotoxic and inhibitory effects on myeloid cells, and clinical effects beyond the principal use for bone metastases have highlighted their immunomodulatory properties.121 Trabectedin was developed as an anti-proliferative agent but was subsequently found to induce significant monocytic cell depletion.122 Further research is needed to better understand the potential of these approaches in cancer therapy.
The enormous heterogeneity and plasticity of macrophages and the vast array of modulatory signals from the microenvironment together make successful immunotherapy aimed at targeting macrophages a complex and difficult approach to navigate. The omnipresence of macrophages and their power to destroy tumour cells, however, make attempts in this field of ‘myeloid-immunotherapy’ worthwhile.
Neutrophil granulocytes
Together with MDSCs123 and macrophages,124,125 neutrophil granulocytes, which are especially enriched in CRC, form a complex network of phagocytosing and immunomodulatory immune cells.126,127 Similar to macrophages, difficulties in the classification and functional characterisation of these cells make directed interventions difficult; however, it is clear that multiple interventions (including GM-CSF, VEGF and chemokine inhibition) can modulate these cells and therefore alter the immunological microenvironment of the tumour. The effect of these interventions is also reflected by changes in the neutrophil-to-lymphocyte ratio, which serves as a secondary biomarker for therapy success in many clinical trials.128
Clinical trials (Table 1) that modulate this group of immune cells are numerous; one such example for neutrophil and MDSC targeting is the inhibition of arginase (produced by these cells), which subsequently leads to T-cell activation.118,130,131,132,133,134 Interestingly, higher densities of tumour-associated neutrophils were associated with better prognosis in CRC134 and, even more surprising, with a better response to fluoracil‐based chemotherapy. Nevertheless, the robust quantification and localisation of neutrophil granulocytes in tissues is still a challenge, again similar to the situation for macrophages.135,136,137
NK cells
NK cells are a subtype of innate lymphoid cell; they are therapeutically attractive owing to their capacity to kill tumour cells without requiring further ‘education’ by other immune cells. It has become clear that there are far more regulatory (and inhibitory) mechanisms within the microenvironment of solid tumours than expected, and studies of CRC and breast cancer have identified that infiltrating NK cells can be selectively suppressed.18,21 Activating and inhibiting receptors, such as killer cell immunoglobulin-like receptors, together with their ligands, form an intricate network that regulates NK cells138,139,140,141 and consequently offer potential for translational intervention. Therefore, aside from the potential to modulate NK cell activation or inactivation in the clinic (Table 1), approaches involving cellular therapies have gained more attraction and trials are underway to evaluate the potential for NK cells in CRC. Of note, many checkpoint inhibitor therapies not only influence effector T cells but also NK cells. The pathway and magnitude of NK cell modulation (via, e.g., PD-1, 4-1BB, CD27, etc.) are poorly understood and the parameters for further combinations and selection of defined patient cohorts are therefore being evaluated.142,143
Fibroblasts
Besides their structural role in tissues, fibroblasts also have a fundamental immunological role, especially with respect to modulation of the innate immune system.144,145 Their inflammatory potential together with their orchestrating function (e.g., via chemokines) make these cells an important immunologic interface. Current clinical trials are either aimed at the destruction (e.g., by targeting fibroblast-activating protein α) or the modulation of fibroblast function; the latter can be achieved by modulating key signalling pathways, including those involving fibroblast growth factor, platelet-derived growth factor, or stromal-derived factor 1α/CXCL12. Clinical trials in overlapping functional areas (e.g., inhibition of angiogenesis and stromal modulation) are common; afatinib provides an example of this, as it targets the stromal compartment and stroma formation. Furthermore, CXCL12 inhibition in cancer-associated fibroblasts showed effects in preclinical models,146 with results indicating that modulation of this axis would abrogate anti-migratory effects, leading to an influx of T cells and tumour cell attack.
The microbiome and modulation of innate immunity in CRC
Survival of the human body depends on tight control of the microbiota, particularly in the gut, and the prevention of unwanted infections. Intestinal epithelial cells are equipped with a vast array of innate immune receptors, highlighting the intimate interplay between the gut content and the immune system.147 Furthermore, signalling by TLRs—among other molecules—is an important pathway in regulating innate immune activation and involves proteins such as MyD88, TNF-associated factor 6 and nuclear factor-κB.148,149 Dysregulation of this pathway can lead to autoimmunity (e.g., colitis or chronic IBD) or neoplastic transformation.9,150,151
Alterations in the composition and localisation of distinct bacterial species within the gut can disturb the equilibrium with the innate immune system. Certain bacteria (e.g., Helicobacter hepaticus) can promote carcinogenesis directly by producing reactive oxygen species, whereas others (e.g., Fusobacterium nucleatum) induce complex immunomodulation that supports the tumour.152,153,154 Furthermore, it was recently shown that the microbiome can shape the response to immunotherapy.155,156,157 The effects of the microbiome on the adaptive arm of the immune system have been described extensively, but very little is known about the bacterial species, effector molecules and molecular regulation through which the microbiome modulates the innate arm of the immune system.158 As described above, there has so far been limited success in immunotherapy for CRC and our understanding of the microbiome and its therapeutic potential in altering the innate immune system is still in its infancy. However, one approach includes the application of probiotics to modify the composition of the bacterial species that are present in the gut of cancer patients and thereby not only ‘correct’ the microbiome but also induce favourable clinical effects for immunotherapies or the course of the disease altogether. This attempt is extremely complex due not only to the lack of a definition of a ‘beneficial’ microbiome for an individual patient, but also due to technical issues of (prolonged) ‘implantation’ of a new microbiome into a patient.159 This approach has therefore only reached entry level for clinical use.160,161,162 It remains to be seen how these observations can be exploited for CRC.
Optimisation of immunotherapy: innate and adaptive immunity together
Careful analyses of the immunological parameters of the local microenvironment have revealed the presence of multiple complex regulatory systems at the tissue level.46,163,164,165,166 The local microenvironment in different organ sites, particularly in metastatic disease, needs to be targeted specifically to enable immunotherapy to be successful. Furthermore, data from clinical trials and limited preclinical models underscore the interdependency between the innate immune system and the adaptive immune response. We need to ‘reprogramme’ the innate immune system, in order to allow long-lasting effector-lymphocyte tumour cell killing; to reach this stage, a greater understanding is required of the tissue-level complexities for the underlying immunological mechanisms, including migration, differentiation, plasticity, adjuvanticity and anti-tumoural functionality. These interdependent systems within the tissue require careful analysis and an improvement in our understanding of the dynamics behind the situations we observe in the clinic.
The role of interventions in the preventive setting also need to be better understood, with data from the systematic use of aspirin and other medications, suggesting a preventive role for certain medications in inhibiting tumour growth and initiation via modulation of immunological parameters.167,168 Yet, given the abundance of clinical evidence, the use of aspirin and its modulatory role in established CRC are not reflected in the current trial landscape, which is an obvious paradox. To escape this shortcoming, a better understanding of the complexities of the immunobiology of (metastatic) CRC with implications for therapeutic combinations and decision making is paramount. Metastatic CRC is not a disease of one system; rather, it comprises multiple diseased systems within a patient and better tools—including multiplex imaging, proteomics, computer modelling169 and others—are needed to fully understand the underlying networks.170,171 The development of parallel links between early-phase clinical trials and biopsy tissue samples is an emerging aspect; given the differences between the biology of the innate immune system in humans and in animals, analysis of human material from clinical trials will be fundamental in ensuring successful therapeutic developments.
Conclusion
The adaptive and innate arms of the immune system are interlocking systems, tightly regulated to protect the human body and maintain integrity, and influencing all possible aspects of cellular regulation; immunological pathways are only one aspect of this regulation. In metastatic disease particularly, we observe a highly specialised network of exploitation, with selective pressure leading to this new cellular composition at the metastatic site. Far from supporting the patient, this microenvironment is optimised for survival of the tumour cells and any interventions will need to overcome the specific regulatory networks responsible. Our existing understanding of the innate arm of the immune system needs to be improved rapidly to devise synergistic and effective clinical strategies. For immunotherapy in solid (metastatic) tumours, synergies between the adaptive and innate arms of the immune system can clearly be harnessed to enhance the anti-tumoural response. In this setting, the precise regulation and timing that govern the activation of the innate immune system are still poorly understood. Data from animal models and clinical trials have indicated an obvious need to better understand the intricate networks of the innate immune system in different affected organs and at different time points during the disease (e.g., localised disease vs. progressive metastatic disease). New models might help to understand the intricacies of the different cellular phenotypes of innate immune system components; understanding the local composition of these cells is key for the application of strategies that target the innate arm as successful immunotherapies in the clinic.
References
Becht E., de Reynies A., Giraldo N. A., Pilati C., Buttard B., Lacroix L. et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin. Cancer Res. 22, 4057–4066 (2016).
Becht, E., Giraldo, N. A., Germain, C., de Reynies, A., Laurent-Puig, P., Zucman-Rossi, J. et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv. Immunol. 130, 95–190 (2016).
Becht, E., Giraldo, N. A., Dieu-Nosjean, M. C., Sautes-Fridman, C. & Fridman, W. H. Cancer immune contexture and immunotherapy. Curr. Opin. Immunol. 39, 7–13 (2016).
DeNardo, D. G., Brennan, D. J., Rexhepaj, E., Ruffell, B., Shiao, S. L., Madden, S. F. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011).
de Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 6, 24–37 (2006).
Basile, D., Garattini, S. K., Bonotto, M., Ongaro, E., Casagrande, M., Cattaneo, M. et al. Immunotherapy for colorectal cancer: where are we heading? Expert. Opin. Biol. Ther. 17, 709–721 (2017).
Coussens, L. M., Zitvogel, L. & Palucka, A. K. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339, 286–291 (2013).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Grivennikov, S. I., Wang, K., Mucida, D., Stewart, C. A., Schnabl, B., Jauch, D. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Brenner, C., Galluzzi, L., Kepp, O. & Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 59, 583–594 (2013).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Balkwill, F. R. & Mantovani, A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 (2012).
Galluzzi, L., Vacchelli, E., Bravo-San Pedro, J. M., Buque, A., Senovilla, L., Baracco, E. E. et al. Classification of current anticancer immunotherapies. Oncotarget 5, 12472–12508 (2014).
Xue, J., Schmidt, S. V., Sander, J., Draffehn, A., Krebs, W., Quester, I. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Beyer, M., Mallmann, M. R., Xue, J., Staratschek-Jox, A., Vorholt, D., Krebs, W. et al. High-resolution transcriptome of human macrophages. PLoS ONE 7, e45466 (2012).
Schroder, K., Irvine, K. M., Taylor, M. S., Bokil, N. J., Le Cao, K. A., Masterman, K. A. et al. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc. Natl Acad. Sci. USA 109, E944–E953 (2012).
Shay, T., Jojic, V., Zuk, O., Rothamel, K., Puyraimond-Zemmour, D., Feng, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl Acad. Sci. USA 110, 2946–2951 (2013).
Halama, N., Braun, M., Kahlert, C., Spille, A., Quack, C., Rahbari, N. et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin. Cancer Res. 17, 678–689 (2011).
Mamessier, E., Bourgin, C. & Olive, D. When breast cancer cells start to fend the educational process of NK cells off. Oncoimmunology 2, e26688 (2013).
Mamessier, E., Bertucci, F., Sabatier, R., Birnbaum, D. & Olive, D. “Stealth” tumors: breast cancer cells shun NK-cells anti-tumor immunity. Oncoimmunology 1, 366–368 (2012).
Mamessier, E., Sylvain, A., Bertucci, F., Castellano, R., Finetti, P., Houvenaeghel, G. et al. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 71, 6621–6632 (2011).
Franklin, R. A., Liao, W., Sarkar, A., Kim, M. V., Bivona, M. R., Liu, K. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Saeed, S., Quintin, J., Kerstens, H. H., Rao, N. A., Aghajanirefah, A., Matarese, F. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 27, 462–472 (2015).
Koliaraki, V., Pallangyo, C. K., Greten, F. R. & Kollias, G. Mesenchymal cells in colon cancer. Gastroenterology 152, 964–979 (2017).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Halama, N., Spille, A., Lerchl, T., Brand, K., Herpel, E., Welte, S. et al. Hepatic metastases of colorectal cancer are rather homogeneous but differ from primary lesions in terms of immune cell infiltration. Oncoimmunology 2, e24116 (2013).
Bryant C. E., Sutherland S., Kong B., Papadimitrious M. S., Fromm P. D., Hart D. N. J. Dendritic cells as cancer therapeutics. Semin. Cell Dev. Biol. 86, 77–88 (2018).
Marin-Acevedo, J. A., Soyano, A. E., Dholaria, B., Knutson, K. L. & Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 11, 8 (2018).
Santos, P. M. & Butterfield, L. H. Dendritic cell-based cancer vaccines. J. Immunol. 200, 443–449 (2018).
Rusakiewicz S., Semeraro M., Sarabi M., Desbois M., Locher C., Mendez R., et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 73, 3499–3510 (2013).
Halama, N., Michel, S., Kloor, M., Zoernig, I., Benner, A., Spille, A. et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 71, 5670–5677 (2011).
Halama, N., Zoernig, I., Spille, A., Michel, S., Kloor, M., Grauling-Halama, S. et al. Quantification of prognostic immune cell markers in colorectal cancer using whole slide imaging tumor maps. Anal. Quant. Cytol. Histol. 32, 333–340 (2010).
Halama, N., Michel, S., Kloor, M., Zoernig, I., Pommerencke, T., von Knebel Doeberitz, M. et al. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 9, 1 (2009).
Halama, N., Zoernig, I., Spille, A., Westphal, K., Schirmacher, P., Jaeger, D. et al. Estimation of immune cell densities in immune cell conglomerates: an approach for high-throughput quantification. PLoS ONE 4, e7847 (2009).
Tanis, E., Julie, C., Emile, J. F., Mauer, M., Nordlinger, B., Aust, D. et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur. J. Cancer 51, 2708–2717 (2015).
Pages, F., Galon, J., Dieu-Nosjean, M. C., Tartour, E., Sautes-Fridman, C. & Fridman, W. H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Mlecnik, B., Bindea, G., Angell, H. K., Maby, P., Angelova, M., Tougeron, D. et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44, 698–711 (2016).
Bindea, G., Mlecnik, B., Tosolini, M., Kirilovsky, A., Waldner, M., Obenauf, A. C. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Galon, J., Angell, H. K., Bedognetti, D. & Marincola, F. M. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39, 11–26 (2013).
Osoegawa, A., Kometani, T., Fukuyama, S., Hirai, F., Seto, T., Sugio, K. et al. Prognostic factors for survival after resection of pulmonary metastases from colorectal carcinoma. Ann. Thorac. Cardiovasc. Surg. 22, 6–11 (2016). PubMed PMID: 26289631.
Blazer, D. G. 3rd, Kishi, Y., Maru, D. M., Kopetz, S., Chun, Y. S., Overman, M. J. et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 26, 5344–5351 (2008).
Nakagawa, K., Tanaka, K., Homma, Y., Nojiri, K., Kumamoto, T., Takeda, K. et al. Low infiltration of peritumoral regulatory T cells predicts worse outcome following resection of colorectal liver metastases. Ann. Surg. Oncol. 22, 180–186 (2015).
Salama, P., Phillips, M., Grieu, F., Morris, M., Zeps, N., Joseph, D. et al. Tumor-infiltrating FOXP3+T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 27, 186–192 (2009).
Halama, N., Zoernig, I., Berthel, A., Kahlert, C., Klupp, F., Suarez-Carmona, M. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 29, 587–601 (2016).
Sherwood, A. M., Emerson, R. O., Scherer, D., Habermann, N., Buck, K., Staffa, J. et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol. Immunother. 62, 1453–1461 (2013).
Bauer, K., Nelius, N., Reuschenbach, M., Koch, M., Weitz, J., Steinert, G. et al. T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunol. Immunother. 62, 27–37 (2013).
Berntsson, J., Nodin, B., Eberhard, J., Micke, P. & Jirstrom, K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int. J. Cancer 139, 1129–1139 (2016).
Xu, W., Joo, H., Clayton, S., Dullaers, M., Herve, M. C., Blankenship, D. et al. Macrophages induce differentiation of plasma cells through CXCL10/IP-10. J. Exp. Med. 209, 1813-23–S1-2 (2012).
Posch, F., Silina, K., Leibl, S., Mundlein, A., Moch, H., Siebenhuner, A. et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 7, e1378844 (2018).
Schweiger, T., Berghoff, A. S., Glogner, C., Glueck, O., Rajky, O., Traxler, D. et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin. Exp. Metastasis. 33, 727–739 (2016).
Di Caro, G., Castino, G. F., Bergomas, F., Cortese, N., Chiriva-Internati, M., Grizzi, F. et al. Tertiary lymphoid tissue in the tumor microenvironment: from its occurrence to immunotherapeutic implications. Int. Rev. Immunol. 34, 123–133 (2015).
Di Caro, G., Bergomas, F., Grizzi, F., Doni, A., Bianchi, P., Malesci, A. et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 20, 2147–2158 (2014).
Nagorsen, D. & Thiel, E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin. Cancer Res. 12, 3064–3069 (2006).
Sasada, T., Kibe, S., Akagi, Y. & Itoh, K. Personalized peptide vaccination for advanced colorectal cancer. Oncoimmunology 4, e1005512 (2015).
Burgdorf, S. K. Dendritic cell vaccination of patients with metastatic colorectal cancer. Dan. Med. Bull. 57, B4171 (2010).
Sahin, U. & Tureci, O. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Parkhurst, M. R., Yang, J. C., Langan, R. C., Dudley, M. E., Nathan, D. A., Feldman, S. A. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Tran, E., Robbins, P. F., Lu, Y. C., Prickett, T. D., Gartner, J. J., Jia, L. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Le, D. T., Uram, J. N., Wang, H., Bartlett, B. R., Kemberling, H., Eyring, A. D. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Chung, K. Y., Gore, I., Fong, L., Venook, A., Beck, S. B., Dorazio, P. et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J. Clin. Oncol. 28, 3485–3490 (2010).
Brahmer, J. R., Drake, C. G., Wollner, I., Powderly, J. D., Picus, J., Sharfman, W. H. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Overman, M. J., McDermott, R., Leach, J. L., Lonardi, S., Lenz, H. J., Morse, M. A. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Patnaik, A., Kang, S. P., Rasco, D., Papadopoulos, K. P., Elassaiss-Schaap, J., Beeram, M. et al. Phase I study of Pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 21, 4286–4293 (2015).
Chalmers, Z. R., Connelly, C. F., Fabrizio, D., Gay, L., Ali, S. M., Ennis, R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Bendell J. C., Powderly, J. D., Lieu, C. H., Eckhardt, S. G., Hurwitz, H., Hochster, H. S., Murphy, J. E., Funke, R. P., Rossi, C., Wallin, J. et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 33, (2015).
Kim, T. K., Herbst, R. S. & Chen, L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 39, 624–631 (2018).
Kather J. N., Halama N. & Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin. Cancer Biol. 152, 189–197 (2018)
Arlauckas S. P., Garris C. S., Kohler R. H., Kitaoka M., Cuccarese M. F., Yang K. S., et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 9, pii: eaal3604 (2017).
Correale, P., Botta, C., Martino, E. C., Ulivieri, C., Battaglia, G., Carfagno, T. et al. Phase Ib study of poly-epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo-immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology 5, e1101205 (2016).
Correale, P., Botta, C., Rotundo, M. S., Guglielmo, A., Conca, R., Licchetta, A. et al. Gemcitabine, oxaliplatin, levofolinate, 5-fluorouracil, granulocyte-macrophage colony-stimulating factor, and interleukin-2 (GOLFIG) versus FOLFOX chemotherapy in metastatic colorectal cancer patients: the GOLFIG-2 multicentric open-label randomized phase III trial. J. Immunother. 37, 26–35 (2014).
McCaw, T. R., Randall, T. D., Forero, A. & Buchsbaum, D. J. Modulation of antitumor immunity with histone deacetylase inhibitors. Immunotherapy 9, 1359–1372 (2017).
Robert, L., Ribas, A. & Hu-Lieskovan, S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Semin. Immunol. 28, 73–80 (2016).
Drew, D. A., Cao, Y. & Chan, A. T. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat. Rev. Cancer 16, 173–186 (2016).
Fletcher, R. H. Review: Aspirin reduces colorectal cancer incidence and mortality in patients at average risk. Ann. Intern. Med. 165, JC16 (2016).
Rothwell, P. M., Wilson, M., Elwin, C. E., Norrving, B., Algra, A., Warlow, C. P. et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376, 1741–1750 (2010).
Flossmann, E. & Rothwell, P. M. British Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369, 1603–1613 (2007).
Umar, A., Steele, V. E., Menter, D. G. & Hawk, E. T. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin. Oncol. 43, 65–77 (2016).
Zumwalt, T. J., Wodarz, D., Komarova, N. L., Toden, S., Turner, J., Cardenas, J. et al. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev. Res. 10, 208–218 (2017).
Gu, M., Nishihara, R., Chen, Y., Li, W., Shi, Y., Masugi, Y. et al. Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells. Oncotarget 8, 87379–87389 (2017).
Turturro, S. B., Najor, M. S., Ruby, C. E., Cobleigh, M. A. & Abukhdeir, A. M. Mutations in PIK3CA sensitize breast cancer cells to physiologic levels of aspirin. Breast Cancer Res. Treat. 156, 33–43 (2016).
Cao, Y., Nishihara, R., Qian, Z. R., Song, M., Mima, K., Inamura, K. et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology 151, 879–92 e4 (2016).
Grau, M. V., Baron, J. A., Sandler, R. S., Haile, R. W., Beach, M. L., Church, T. R. et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J. Natl Cancer Inst. 95, 1765–1771 (2003).
Song, M., Nishihara, R., Wang, M., Chan, A. T., Qian, Z. R., Inamura, K. et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 65, 296–304 (2016).
Kundu, R., Theodoraki, A., Haas, C. T., Zhang, Y., Chain, B., Kriston-Vizi, J. et al. Cell-type-specific modulation of innate immune signalling by vitamin D in human mononuclear phagocytes. Immunology 150, 55–63 (2017).
Song, M., Zhang, X., Meyerhardt, J. A., Giovannucci, E. L., Ogino, S., Fuchs, C. S. et al. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 66, 1790–1796 (2017).
Kopp, T. I., Vogel, U., Tjonneland, A. & Andersen, V. Meat and fiber intake and interaction with pattern recognition receptors (TLR1, TLR2, TLR4, and TLR10) in relation to colorectal cancer in a Danish prospective, case-cohort study. Am. J. Clin. Nutr. 107, 465–479 (2018).
Tian, Y., Xu, Q., Sun, L., Ye, Y. & Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 57, 103–109 (2018).
Kirwan, A. M., Lenighan, Y. M., O’Reilly, M. E., McGillicuddy, F. C. & Roche, H. M. Nutritional modulation of metabolic inflammation. Biochem. Soc. Trans. 45, 979–985 (2017).
Alvarez-Curto, E. & Milligan, G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 114, 3–13 (2016).
Keim, S., Zoernig, I., Spille, A., Lahrmann, B., Brand, K., Herpel, E. et al. Sequential metastases of colorectal cancer: Immunophenotypes and spatial distributions of infiltrating immune cells in relation to time and treatments. Oncoimmunology 1, 593–599 (2012).
Algars, A., Irjala, H., Vaittinen, S., Huhtinen, H., Sundstrom, J., Salmi, M. et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int. J. Cancer 131, 864–873 (2012).
Klug, F., Prakash, H., Huber, P. E., Seibel, T., Bender, N., Halama, N. et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 24, 589–602 (2013).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Zhang, C., Wang, Y., Wang, F., Wang, Z., Lu, Y., Xu, Y. et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci. Rep. 7, 412 (2017).
Gu, W., Yao, L., Li, L., Zhang, J., Place, A. T., Minshall, R. D. et al. ICAM-1 regulates macrophage polarization by suppressing MCP-1 expression via miR-124 upregulation. Oncotarget 8, 111882–111901 (2017).
Colegio, O. R., Chu, N. Q., Szabo, A. L., Chu, T., Rhebergen, A. M., Jairam, V. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Grossardt, C., Engeland, C. E., Bossow, S., Halama, N., Zaoui, K., Leber, M. F. et al. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 24, 644–654 (2013).
Sunakawa, Y., Stintzing, S., Cao, S., Heinemann, V., Cremolini, C., Falcone, A. et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann. Oncol. 26, 2450–2456 (2015).
Daemen, T., Regts, J., Morselt, H. & Scherphof, G. L. The effect of liver macrophages on in vitro cytolytic activity of 5FU and FUdR on colon carcinoma cells: evidence of macrophage activation. Int. J. Immunopharmacol. 14, 857–864 (1992).
Malesci, A., Bianchi, P., Celesti, G., Basso, G., Marchesi, F., Grizzi, F. et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology 6, e1342918 (2017).
Aras, S. & Zaidi, M. R. TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 117, 1583–1591 (2017).
Rojas, J., Salazar, J., Martinez, M. S., Palmar, J., Bautista, J., Chavez-Castillo, M. et al. Macrophage heterogeneity and plasticity: impact of macrophage biomarkers on atherosclerosis. Scientifica 2015, 851252 (2015).
Edin, S., Wikberg, M. L., Dahlin, A. M., Rutegard, J., Oberg, A., Oldenborg, P. A. et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE 7, e47045 (2012).
Illemann, M., Laerum, O. D., Hasselby, J. P., Thurison, T., Hoyer-Hansen, G., Nielsen, H. J. et al. Urokinase-type plasminogen activator receptor (uPAR) on tumor-associated macrophages is a marker of poor prognosis in colorectal cancer. Cancer Med. 3, 855–864 (2014).
Kinouchi, M., Miura, K., Mizoi, T., Ishida, K., Fujibuchi, W., Ando, T. et al. Infiltration of CD14-positive macrophages at the invasive front indicates a favorable prognosis in colorectal cancer patients with lymph node metastasis. Hepato Gastroenterol. 58, 352–358 (2011).
Kinouchi, M., Miura, K., Mizoi, T., Ishida, K., Fujibuchi, W., Sasaki, H. et al. Infiltration of CD40-positive tumor-associated macrophages indicates a favorable prognosis in colorectal cancer patients. Hepato Gastroenterol. 60, 83–88 (2013).
Nywening, T. M., Wang-Gillam, A., Sanford, D. E., Belt, B. A., Panni, R. Z., Cusworth, B. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016).
Papadopoulos, K. P., Gluck, L., Martin, L. P., Olszanski, A. J., Tolcher, A. W., Ngarmchamnanrith, G. et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res. 23, 5703–5710 (2017).
Ries, C. H., Cannarile, M. A., Hoves, S., Benz, J., Wartha, K., Runza, V. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 25, 846–859 (2014).
Bronte, V. & Bria, E. Interfering with CCL5/CCR5 at the tumor-stroma interface. Cancer Cell. 29, 437–439 (2016).
Halama N. CCR5 inhibition in colorectal cancer patients. Transl. Cancer Re. 5, S366–S367 (2016).
Halama, N. Macrophage repolarisation therapy in colorectal cancer. ESMO Open 3, e000426 (2018).
Bruchard, M., Mignot, G., Derangere, V., Chalmin, F., Chevriaux, A., Vegran, F. et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 19, 57–64 (2013).
Luddy, K. A., Robertson-Tessi, M., Tafreshi, N. K., Soliman, H., Morse, D. L. The role of toll-like receptors in colorectal cancer progression: evidence for epithelial to leucocytic transition. Front. Immunol. 5, 429 (2014).
Dalgleish, A. G. Rationale for combining immunotherapy with chemotherapy. Immunotherapy 7, 309–316 (2015).
Ebert, P. J., Cheung, J., Yang, Y., McNamara, E., Hong, R., Moskalenko, M. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Bendell, J., Cardiello, F., Tabernero, J., Tebbutt, N., Eng, C., Di Bartolomeo, M. et al. Efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab+cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Ann. Oncol. 29, mdy208.003 (2018).
Diel, I. J., Solomayer, E. F., Costa, S. D., Gollan, C., Goerner, R., Wallwiener, D. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998).
Germano, G., Frapolli, R., Belgiovine, C., Anselmo, A., Pesce, S., Liguori, M. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 23, 249–262 (2013).
Talmadge, J. E. & Gabrilovich, D. I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13, 739–752 (2013).
Mantovani, A. & Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 212, 435–445 (2015).
Mantovani, A., Vecchi, A. & Allavena, P. Pharmacological modulation of monocytes and macrophages. Curr. Opin. Pharmacol. 17, 38–44 (2014).
Schlecker, E., Stojanovic, A., Eisen, C., Quack, C., Falk, C. S., Umansky, V. et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 189, 5602–5611 (2012).
Umansky, V., Blattner, C., Gebhardt, C. & Utikal, J. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol. Immunother. 66, 1015–1023 (2017).
Song, Y., Yang, Y., Gao, P., Chen, X., Yu, D., Xu, Y. et al. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. Bmc. Cancer 17, 744 (2017).
Orillion, A., Hashimoto, A., Damayanti, N., Shen, L., Adelaiye-Ogala, R., Arisa, S. et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin. Cancer Res. 23, 5187–5201 (2017).
Antonia, S. J., Vansteenkiste, J. F. & Moon, E. Immunotherapy: beyond anti-PD-1 and anti-PD-L1 therapies. Am. Soc. Clin. Oncol. 35, e450–e458 (2016).
Rotondo, R., Barisione, G., Mastracci, L., Grossi, F., Orengo, A. M., Costa, R. et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int. J. Cancer 125, 887–893 (2009).
Rodriguez, P. C., Quiceno, D. G., Zabaleta, J., Ortiz, B., Zea, A. H., Piazuelo, M. B. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Munder, M., Eichmann, K., Moran, J. M., Centeno, F., Soler, G. & Modolell, M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 163, 3771–3777 (1999).
Galdiero, M. R., Bianchi, P., Grizzi, F., Di Caro, G., Basso, G., Ponzetta, A. et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer 139, 446–456 (2016).
Zhou, G., Peng, K., Song, Y., Yang, W., Shu, W., Yu, T. et al. CD177+neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis 39, 272–282 (2018).
Berry, R. S., Xiong, M. J., Greenbaum, A., Mortaji, P., Nofchissey, R. A., Schultz, F. et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One 12, e0188799 (2017).
Schleypen, J. S., Baur, N., Kammerer, R., Nelson, P. J., Rohrmann, K., Grone, E. F. et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 12(3 Pt 1), 718–725 (2006).
Schleypen, J. S., Von Geldern, M., Weiss, E. H., Kotzias, N., Rohrmann, K., Schendel, D. J. et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int. J. Cancer 106, 905–912 (2003).
Sers, C., Kuner, R., Falk, C. S., Lund, P., Sueltmann, H., Braun, M. et al. Down-regulation of HLA Class I and NKG2D ligands through a concerted action of MAPK and DNA methyltransferases in colorectal cancer cells. Int. J. Cancer 125, 1626–1639 (2009).
Anfossi, N., Andre, P., Guia, S., Falk, C. S., Roetynck, S., Stewart, C. A. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006).
Johnston, R. J., Comps-Agrar, L., Hackney, J., Yu, X., Huseni, M., Yang, Y. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 26, 923–937 (2014).
Morvan, M. G. & Lanier, L. L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16, 7–19 (2016).
Zhou, W., Xu, G., Wang, Y., Xu, Z., Liu, X., Xu, X. et al. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle 16, 73–81 (2017).
Ahmed, M. A., Selzer, E., Dorr, W., Jomrich, G., Harpain, F., Silberhumer, G. R. et al. Fibroblast growth factor receptor 4 induced resistance to radiation therapy in colorectal cancer. Oncotarget 7, 69976–69990 (2016).
Feig, C., Jones, J. O., Kraman, M., Wells, R. J., Deonarine, A., Chan, D. S. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Pott, J. & Hornef, M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 13, 684–698 (2012).
Rakoff-Nahoum, S., Hao, L. & Medzhitov, R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity 25, 319–329 (2006).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Srivatsa, S., Paul, M. C., Cardone, C., Holcmann, M., Amberg, N., Pathria, P. et al. EGFR in tumor-associated myeloid cells promotes development of colorectal cancer in mice and associates with outcomes of patients. Gastroenterology 153, 178–90 e10 (2017).
Bergmann, H., Roth, S., Pechloff, K., Kiss, E. A., Kuhn, S., Heikenwalder, M. et al. Card9-dependent IL-1beta regulates IL-22 production from group 3 innate lymphoid cells and promotes colitis-associated cancer. Eur. J. Immunol. 47, 1342–1353 (2017).
Ramos, A. & Hemann, M. T. Drugs, bugs, and cancer: Fusobacterium nucleatum promotes chemoresistance in colorectal cancer. Cell 170, 411–413 (2017).
Yang, Y., Weng, W., Peng, J., Hong, L., Yang, L., Toiyama, Y. et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology 152, 851–66 e24 (2017).
Irrazabal, T., Belcheva, A., Girardin, S. E., Martin, A. & Philpott, D. J. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell 54, 309–320 (2014).
Viaud, S., Daillere, R., Boneca, I. G., Lepage, P., Langella, P., Chamaillard, M. et al. Gut microbiome and anticancer immune response: really hot Sh*t! Cell Death Differ. 22, 199–214 (2015).
Vetizou, M., Pitt, J. M., Daillere, R., Lepage, P., Waldschmitt, N., Flament, C. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Viaud, S., Daillere, R., Boneca, I. G., Lepage, P., Pittet, M. J., Ghiringhelli, F. et al. Harnessing the intestinal microbiome for optimal therapeutic immunomodulation. Cancer Res. 74, 4217–4221 (2014).
Routy, B., Gopalakrishnan, V., Daillere, R., Zitvogel, L., Wargo, J. A. & Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Suez, J., Zmora, N., Zilberman-Schapira, G., Mor, U., Dori-Bachash, M., Bashiardes, S. et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174, 1406–23 e16 (2018).
Golkhalkhali B., Rajandram R., Paliany A. S., Ho G. F., Wan Ishak W. Z., Johari C. S., et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia Pac. J. Clin. Oncol. 14, 171–191 (2017).
Cousin, F. J., Jouan-Lanhouet, S., Theret, N., Brenner, C., Jouan, E., Le Moigne-Muller, G. et al. The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL-based therapy in colorectal cancer. Oncotarget 7, 7161–7178 (2016).
Aisu, N., Tanimura, S., Yamashita, Y., Yamashita, K., Maki, K., Yoshida, Y. et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp. Ther. Med. 10, 966–972 (2015).
Tape, C. J. Systems biology analysis of heterocellular signaling. Trends Biotechnol. 34, 627–637 (2016).
Tape C. J., Ling S., Dimitriadi M., McMahon K. M., Worboys J. D., Leong H. S., et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165, 1818 (2016).
Halama N. The next age of immunotherapy: optimisation, stratification and therapeutic synergies. Br. J. Cancer 120, 12 (2019).
Kather, J. N., Berghoff, A. S., Ferber, D., Suarez-Carmona, M., Reyes-Aldasoro, C. C., Valous, N. A. et al. Large-scale database mining reveals hidden trends and future directions for cancer immunotherapy. Oncoimmunology 7, e1444412 (2018).
Kensler, T. W., Spira, A., Garber, J. E., Szabo, E., Lee, J. J., Dong, Z. et al. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev. Res. 9, 2–10 (2016).
Spira, A., Yurgelun, M. B., Alexandrov, L., Rao, A., Bejar, R., Polyak, K. et al. Precancer atlas to drive precision prevention trials. Cancer Res. 77, 1510–1541 (2017).
Kather J. N., Poleszczuk J., Suarez-Carmona M., Krisam J., Charoentong P., Valous N. A., et al. In silico modeling of immunotherapy and stroma-targeting therapies in human colorectal cancer. Cancer Res. 15, 6442–6452 (2017).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
West, N. R., McCuaig, S., Franchini, F. & Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 15, 615–629 (2015).
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NH drafted and conceived the manuscript and designed the figures. JK critically read and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is a conflict of interest. NH holds intellectual property on the use of CCR5 Inhibition in the Treatment of cancer and is a subject editor at the British Journal of Cancer.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kather, J.N., Halama, N. Harnessing the innate immune system and local immunological microenvironment to treat colorectal cancer. Br J Cancer 120, 871–882 (2019). https://doi.org/10.1038/s41416-019-0441-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0441-6
This article is cited by
-
CAFs secrete CXCL12 to accelerate the progression and cisplatin resistance of colorectal cancer through promoting M2 polarization of macrophages
Medical Oncology (2023)
-
MHBSt167 induced autophagy promote cell proliferation and EMT by activating the immune response in L02 cells
Virology Journal (2022)
-
Immune cell profiles in the tumor microenvironment of early-onset, intermediate-onset, and later-onset colorectal cancer
Cancer Immunology, Immunotherapy (2022)
-
MicroRNA-146a limits tumorigenic inflammation in colorectal cancer
Nature Communications (2021)
-
Stroma-derived ANGPTL2 establishes an anti-tumor microenvironment during intestinal tumorigenesis
Oncogene (2021)