Abstract
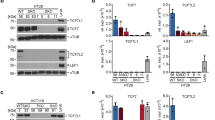
Although significant progress has been made in understanding the importance of Wnt signaling in the initiation of colorectal cancer, less is known about responses that accompany the reversal of oncogenic Wnt signaling. The aim of this study was to analyze in vivo and in vitro responses to an ‘ideal’ Wnt pathway inhibitor as a model for the therapeutic targeting of the pathway. A tetracycline-inducible transgenic mouse model expressing truncated β-catenin (ΔN89β-catenin) that exhibited a strong intestinal hyperplasia was analyzed during the removal of oncogenic β-catenin expression both in 3D ‘crypt culture’ and in vivo. Oncogenic Wnt signaling was rapidly and completely reversed. The strongest inhibition of Wnt target gene expression occurred within 24 h of doxycycline removal at which time the target genes Ascl2, Axin2 and C-myc were downregulated to levels below that in the control intestine. In vitro, the small molecule Wnt inhibitor CCT036477 induced a response within 4 h of treatment. By 7 days following doxycycline withdrawal, gene expression, cell proliferation and tissue morphology were undistinguishable from control animals.In conclusion, these results demonstrate that the reversal of Wnt signaling by inhibitors should ideally be studied within hours of treatment. The reversible system described, involving medium throughput in vitro approaches and rapid in vivo responses, should allow the rapid advance of early stage compounds into efficacy models that are more usually considered later in the drug discovery pipeline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Van der Flier LG, Clevers H . Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009; 71: 241–260.
Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 2002; 16: 1066–1076.
Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M et al. The intestinal Wnt/TCF signature. Gastroenterology 2007; 132: 628–632.
Fevr T, Robine S, Louvard D, Huelsken J . Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007; 27: 7551–7559.
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998; 19: 379–383.
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 2004; 101: 266–271.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265.
Giles RH, van Es JH, Clevers H . Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24.
Polakis P . Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851.
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 1999; 18: 5931–5942.
Wong MH, Rubinfeld B, Gordon JI . Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J Cell Biol 1998; 141: 765–777.
Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M et al. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res 1999; 59: 3875–3879.
Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R . Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 2006; 44: 23–28.
Bocker R, Estler CJ, Maywald M, Weber D . Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittelforschung 1981; 31: 2116–2117.
Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P . Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 1997; 275: 1790–1792.
Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res 2010; 70: 5963–5973.
Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB et al. Identification of FAP locus genes from chromosome 5q21. Science 1991; 253: 661–665.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997; 275: 1787–1790.
Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M et al. Crypt-restricted proliferation and commitment to the paneth cell lineage following Apc loss in the mouse intestine. Development 2005; 132: 1443–1451.
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 2004; 18: 1385–1390.
Logan CY, Nusse R . The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810.
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 2004; 23: 8520–8526.
Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlosser A et al. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol 2009; 82: 165–175.
He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 2004; 6: 7–14.
Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 2011; 108: 17135–17140.
Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev 2003; 17: 488–501.
Coste I, Freund JN, Spaderna S, Brabletz T, Renno T . Precancerous lesions upon sporadic activation of beta-catenin in mice. Gastroenterology 2007; 132: 1299–1308.
Lucero OM, Dawson DW, Moon RT, Chien AJ . A re-evaluation of the ″oncogenic″ nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep 2010; 12: 314–318.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418.
Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA 2007; 104: 14700–14705.
Buchert M, Athineos D, Abud HE, Burke ZD, Faux MC, Samuel MS et al. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS Genet 2010; 6: e1000816.
Wang Y . Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther 2009; 8: 2103–2109.
van Amerongen R, Mikels A, Nusse R . Alternative wnt signaling is initiated by distinct receptors. Sci Signal 2008; 1: re9.
Soriano P . Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21: 70–71.
Wutz A, Jaenisch R . A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell 2000; 5: 695–705.
Shorning BY, Jarde T, McCarthy A, Ashworth A, de Leng WW, Offerhaus GJ et al. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut 2012; 61: 202–213.
Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 2002; 22: 1184–1193.
Merritt AJ, Allen TD, Potten CS, Hickman JA . Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 1997; 14: 2759–2766.
Acknowledgements
This work was supported by Cancer Research UK and the Breast Cancer Campaign. We thank C Beard and R Jaenisch (The Whitehead Institute for Biomedical Research, Massachusetts) for the embryonic stem cell lines and for technical advice; K Ewan, A Offergeld and S Braun (Cardiff School of Biosciences) for their assistance in tissue staining and cell counting; P Watson (Cardiff School of Biosciences) for his assistance in fluorescence microscopy; O Sansom and K Myant for their assistance in organoid culture; B Allen and O Asby for technical assistance in blastocyst injections and chimera crosses; and D Scarborough for help with histology.
Author contributions: TCD, ARC and TJ were involved in the study concept and design; TCD and TJ were involved in funding and study supervision; TJ, RJE, KLM, LP, GJF and BA were involved in acquisition and analysis of data; TJ and TCD were involved in drafting the manuscript; ARC, RJE, LP and GJF were involved in critical revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Jardé, T., Evans, R., McQuillan, K. et al. In vivo and in vitro models for the therapeutic targeting of Wnt signaling using a Tet-OΔN89β-catenin system. Oncogene 32, 883–893 (2013). https://doi.org/10.1038/onc.2012.103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.103
Keywords
This article is cited by
-
Signaling pathways involved in colorectal cancer progression
Cell & Bioscience (2019)
-
Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids
Nature Communications (2016)