Abstract
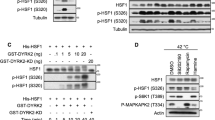
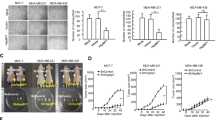
The heat-shock transcription factor HSF1 was recently shown to have a key role in the development of tumors associated with activation of Ras or inactivation of p53. Here, we show that HSF1 is required for the cell transformation and tumorigenesis induced by the human epidermal growth factor receptor-2 (HER2) oncogene responsible for aggressive breast tumors. Upon expression of HER2, untransformed human mammary epithelial MCF-10A cells underwent neoplastic transformation, formed foci in culture and tumors in nude mouse xenografts. However, expression of HER2 in MCF-10A cells with knockdown of HSF1 did not cause either foci formation or tumor growth in xenografts. The antitumorigenic effect of downregulation of HSF1 was associated with HER2-induced accumulation of the cyclin-dependent kinase inhibitor p21 and decrease in the mitotic regulator survivin, which resulted in growth inhibition and cell senescence. In fact, either knockout of p21 or overexpression of survivin alleviated these effects of HSF1 knockdown. The proliferation of certain human HER2-positive breast cancer lines also requires HSF1, as its knockdown led to upregulation of p21 and/or decrease in survivin, precipitating growth arrest. Similar effects were observed with a small-molecular-weight inhibitor of the heat-shock response NZ28. The effects of HSF1 knockdown on the growth arrest and senescence of HER2-expressing cells were associated with downregulation of heat-shock protein (Hsp)72 and Hsp27. Therefore, HSF1 is critical for proliferation of HER2-expressing cells, most likely because it maintains the levels of HSPs, which in turn control regulators of senescence p21 and survivin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Altieri DC . (2003). Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 22: 8581–8589.
Altieri DC . (2008). Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8: 61–70.
Bachman KE, Blair BG, Brenner K, Bardelli A, Arena S, Zhou S et al. (2004). p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther 3: 221–225.
Cahill CM, Lin HS, Price BD, Bruce JL, Calderwood SK . (1997). Potential role of heat shock transcription factor in the expression of inflammatory cytokines. Adv Exp Med Biol 400B: 625–630.
Cahill CM, Waterman WR, Xie Y, Auron PE, Calderwood SK . (1996). Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J Biol Chem 271: 24874–24879.
Cai L, Zhu JD . (2003). The tumor-selective over-expression of the human Hsp70 gene is attributed to the aberrant controls at both initiation and elongation levels of transcription. Cell Res 13: 93–109.
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR . (2006). Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31: 164–172.
Ciocca DR, Calderwood SK . (2005). Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10: 86–103.
Dai C, Whitesell L, Rogers AB, Lindquist S . (2007). Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130: 1005–1018.
Gabai VL, Yaglom JA, Waldman T, Sherman MY . (2009). Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol 29: 559–569.
Jaattela M . (1999). Escaping cell death: survival proteins in cancer. Exp Cell Res 248: 30–43.
Jolly C, Morimoto RI . (2000). Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92: 1564–1572.
Khaleque MA, Bharti A, Gong J, Gray PJ, Sachdev V, Ciocca DR et al. (2008). Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene 27: 1886–1893.
Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA et al. (2005). Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene 24: 6564–6573.
Lacroix M, Toillon RA, Leclercq G . (2006). p53 and breast cancer, an update. Endocr Relat Cancer 13: 293–325.
Lindquist S, Craig EA . (1988). The heat-shock proteins. Annu Rev Genet 22: 631–677.
Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF . (2007). Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene 26: 5086–5097.
Moasser MM . (2007). The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26: 6469–6487.
Morimoto RI, Sarge KD, Abravaya K . (1992). Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem 267: 21987–21990.
Mosser DD, Morimoto RI . (2004). Molecular chaperones and the stress of oncogenesis. Oncogene 23: 2907–2918.
Pratt WB, Toft DO . (2003). Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228: 111–133.
Roninson IB . (2003). Tumor cell senescence in cancer treatment. Cancer Res 63: 2705–2715.
Singh IS, He JR, Calderwood S, Hasday JD . (2002). A high affinity HSF-1 binding site in the 5′-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem 277: 4981–4988.
Singh IS, Viscardi RM, Kalvakolanu I, Calderwood S, Hasday JD . (2000). Inhibition of tumor necrosis factor-alpha transcription in macrophages exposed to febrile range temperature. A possible role for heat shock factor-1 as a negative transcriptional regulator. J Biol Chem 275: 9841–9848.
Sorger PK . (1991). Heat shock factor and the heat shock response. Cell 65: 363–366.
Trost TM, Lausch EU, Fees SA, Schmitt S, Enklaar T, Reutzel D et al. (2005). Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res 65: 840–849.
Wu G, Osada M, Guo Z, Fomenkov A, Begum S, Zhao M et al. (2005). DeltaNp63alpha up-regulates the Hsp70 gene in human cancer. Cancer Res 65: 758–766.
Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK . (2002a). Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem 277: 11802–11810.
Xie Y, Chen C, Stevenson MA, Hume DA, Auron PE, Calderwood SK . (2002b). NF-IL6 and HSF1 have mutually antagonistic effects on transcription in monocytic cells. Biochem Biophys Res Commun 291: 1071–1080.
Yaglom JA, Gabai VL, Sherman MY . (2007). High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res 67: 2373–2381.
Zaarur N, Gabai VL, Porco Jr JA., Calderwood S, Sherman MY . (2006). Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res 66: 1783–1791.
Acknowledgements
This work was supported by grants from the National Institute of Health CA081244. We thank Dr B Park and Dr C Spangenberg for their kind supply of MCF-10A cells and HER2-overexpressing retroviral vector.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Meng, L., Gabai, V. & Sherman, M. Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene 29, 5204–5213 (2010). https://doi.org/10.1038/onc.2010.277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.277
Keywords
This article is cited by
-
Involvement of redox signalling in tumour cell dormancy and metastasis
Cancer and Metastasis Reviews (2023)
-
Gastric cancer exosomes contribute to the field cancerization of gastric epithelial cells surrounding gastric cancer
Gastric Cancer (2022)
-
Thrombospondin 4/integrin α2/HSF1 axis promotes proliferation and cancer stem-like traits of gallbladder cancer by enhancing reciprocal crosstalk between cancer-associated fibroblasts and tumor cells
Journal of Experimental & Clinical Cancer Research (2021)
-
The C-terminal HSP90 inhibitor NCT-58 kills trastuzumab-resistant breast cancer stem-like cells
Cell Death Discovery (2021)
-
The stress-responsive kinase DYRK2 activates heat shock factor 1 promoting resistance to proteotoxic stress
Cell Death & Differentiation (2021)