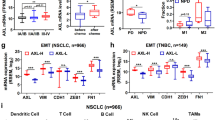
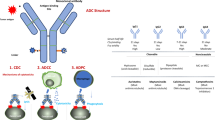
Abstract
Axl is expressed in various types of cancer and is involved in multiple processes of tumorigenesis, including promoting tumor cell growth, migration, invasion, metastasis as well as angiogenesis. To evaluate further the mechanisms involved in the expression/activation of Axl in various aspects of tumorigenesis, especially its roles in modulating tumor stromal functions, we have developed a phage-derived mAb (YW327.6S2) that recognizes both human and murine Axl. YW327.6S2 binds to both human and murine Axl with high affinity. It blocks the ligand Gas6 binding to the receptor, downregulates receptor expression, inhibits receptor activation and downstream signaling. In A549 non-small-cell lung cancer (NSCLC) and MDA-MB-231 breast cancer models, YW327.6S2 attenuates xenograft tumor growth and potentiates the effect of anti-VEGF treatment. In NSCLC models, YW327.6S2 also enhances the effect of erlotinib and chemotherapy in reducing tumor growth. Furthermore, YW327.6S2 reduces the metastasis of MDA-MB-231 breast cancer cells to distant organs. YW327.6S2 induces tumor cell apoptosis in NSCLC, reduces tumor-associated vascular density and inhibits the secretion of inflammatory cytokines and chemokines from tumor-associated macrophages in the breast cancer model. In conclusion, anti-Axl mAb can enhance the therapeutic efficacy of anti-VEGF, EGFR small-molecule inhibitors as well as chemotherapy. Axl mAb affects not only tumor cells but also tumor stroma through its modulation of tumor-associated vasculature and immune cell functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Balkwill F, Charles KA, Mantovani A . (2005). Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7: 211–217.
Balkwill F, Mantovani A . (2001). Inflammation and cancer: back to Virchow? Lancet 357: 539–545.
Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC . (2001). Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol 12: 819–824.
Brey EM, Lalani Z, Johnston C, Wong M, McIntire LV, Duke PJ et al. (2003). Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem 51: 575–584.
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL et al. (1992). Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 89: 4285–4289.
Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW . (2003). Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol 22: 533–540.
Coussens LM, Werb Z . (2002). Inflammation and cancer. Nature 420: 860–867.
Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S et al. (1995). Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer 60: 791–797.
Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS et al. (2005). Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23: 5900–5909.
Giaccone G . (2005). Epidermal growth factor receptor inhibitors in the treatment of non-small-cell lung cancer. J Clin Oncol 23: 3235–3242.
Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T et al. (2010). Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA 107: 1124–1129.
Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB et al. (2000). The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol 67: 53–62.
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W et al. (2010). R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 70: 1544–1554.
Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE et al. (2005). Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res 65: 9294–9303.
Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT et al. (2008). Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 268: 314–324.
Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N et al. (2008). Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res 14: 130–138.
Ito T, Ito M, Naito S, Ohtsuru A, Nagayama Y, Kanematsu T et al. (1999). Expression of the Axl receptor tyrosine kinase in human thyroid carcinoma. Thyroid 9: 563–567.
Janssen JW, Schulz AS, Steenvoorden AC, Schmidberger M, Strehl S, Ambros PF et al. (1991). A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene 6: 2113–2120.
Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ et al. (2009). The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther 8: 618–626.
Lai C, Lemke G . (1991). An extended family of protein-tyrosine linase genes differentially expressed in the vertebrate nervous system. Neuron 6: 691–704.
Lee CV, Liang WC, Dennis MS, Eigenbrot C, Sidhu SS, Fuh G . (2004). High-affinity human antibodies from phage-displayed synthetic Fab libraries with a single framework scaffold. J Mol Biol 340: 1073–1093.
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J et al. (2009). Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 28: 3442–3455.
Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J et al. (2006). Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281: 951–961.
Liang WC, Dennis MS, Stawicki S, Chanthery Y, Pan Q, Chen Y et al. (2007). Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol 366: 815–829.
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R et al. (2009). Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 69: 6871–6878.
Loges S, Schmidt T, Tjwa M, van Geyte K, Lievens D, Lutgens E et al. (2010). Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115: 2264–2273.
Lynch TJ, Bell TW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139.
Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K et al. (2007). A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 26: 3909–3919.
Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC . (2002). Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res 8: 361–367.
O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C et al. (1991). Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11: 5016–5031.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. (2004). EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500.
Pollard JW . (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4: 71–78.
Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H et al. (2007). Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131: 1190–1203.
Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC . (2005). Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol 204: 36–44.
Sandley A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al. (2006). Palitaxel–carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med 355: 2542–2550.
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS et al. (2005). Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 7: 1058–1064.
Sidhu SS, Li B, Chen Y, Fellouse FA, Eigenbrot C, Fuh G . (2004). Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions. J Mol Biol 338: 299–310.
Sun W, Fujimoto J, Tamaya T . (2004). Coexpression of Gas6/Axl in human ovarian cancers. Oncology 66: 450–457.
Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW . (2008). Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene 27: 4044–4055.
Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J et al. (2005). Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med 353: 133–144.
Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H et al. (2006). Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA 103: 5799–5804.
Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS et al. (2002). Clinical significance of AXL kinase family in gastric cancer. Anticancer Res 22: 1071–1078.
Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L et al. (2005). Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 11: 8686–8698.
Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L et al. (2008). AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res 68: 1905–1915.
Acknowledgements
We thank Dr Jiping Zha for developing the dual anti-Axl/CD68 IHC protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. The authors are all employees of Genentech.
Rights and permissions
About this article
Cite this article
Ye, X., Li, Y., Stawicki, S. et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29, 5254–5264 (2010). https://doi.org/10.1038/onc.2010.268
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.268
Keywords
This article is cited by
-
TAM family kinases as therapeutic targets at the interface of cancer and immunity
Nature Reviews Clinical Oncology (2023)
-
Inhibition of AXL receptor tyrosine kinase enhances brown adipose tissue functionality in mice
Nature Communications (2023)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2023)
-
AXL Inhibitors: Status of Clinical Development
Current Oncology Reports (2023)
-
AXL regulates neuregulin1 expression leading to cetuximab resistance in head and neck cancer
BMC Cancer (2022)