Abstract
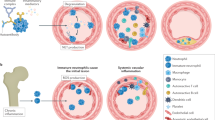
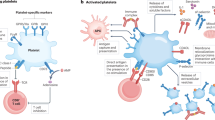
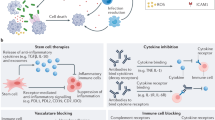
Microparticles (MPs) are small membrane-bound vesicles that are emerging as important elements in the pathogenesis of rheumatic diseases owing to their pleiotropic effects on thrombosis, vascular reactivity, angiogenesis and inflammation. Released from cells during activation and apoptosis, MPs carry proteins, lipids and nucleic acids, and serve as platforms for enzymatic processes in thrombosis. Furthermore, MPs can transfer cytokines, receptors, RNA and DNA to modulate the properties of target cells. As MPs appear in the blood in increased numbers during rheumatic disease, they represent novel biomarkers that can be used to assess events in otherwise inaccessible tissues. Future research will define further the pathogenetic role of MPs and explore therapeutic strategies to block their release or signaling properties.
Key Points
-
Microparticles (MPs) are membrane-bound vesicles that are released from cells during cell activation and apoptosis
-
Depending on the cellular origin and the process of their release, MPs can promote hemostasis, vascular reactivity, angiogenesis and immunity
-
Levels of MPs are increased in the blood of patients with rheumatic diseases, suggesting an important role in disease pathogenesis
-
Circulating MPs represent novel biomarkers for otherwise inaccessible tissues (e.g. endothelium)
-
To establish MPs as biomarkers in rheumatic diseases, future studies need to standardize techniques for isolation and quantification
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ardoin, S. P., Shanahan, J. C. & Pisetsky, D. S. The role of microparticles in inflammation and thrombosis. Scand. J. Immunol. 66, 159–165 (2007).
Hugel, B., Martinez, M. C., Kunzelmann, C. & Freyssinet, J. M. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 20, 22–27 (2005).
Distler, J. H. et al. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 52, 3337–3348 (2005).
Cocucci, E., Racchetti, G. & Meldolesi, J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51 (2009).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Belting, M. & Wittrup, A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J. Cell Biol. 183, 1187–1191 (2008).
Hristov, M., Erl, W., Linder, S. & Weber, P. C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104, 2761–2766 (2004).
Kobayashi, T., Okamoto, H., Yamada, J., Setaka, M. & Kwan, T. Vesiculation of platelet plasma membranes. Dilauroylglycerophosphocholine-induced shedding of a platelet plasma membrane fraction enriched in acetylcholinesterase activity. Biochim. Biophys. Acta 778, 210–218 (1984).
Fox, J. E., Austin, C. D., Boyles, J. K. & Steffen, P. K. Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J. Cell Biol. 111, 483–493 (1990).
Piccin, A., Murphy, W. G. & Smith, O. P. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21, 157–171 (2007).
McLaughlin, P. J., Gooch, J. T., Mannherz, H. G. & Weeds, A. G. Structure of gelsolin segment 1–actin complex and the mechanism of filament severing. Nature 364, 685–692 (1993).
Connor, J., Pak, C. H., Zwaal, R. F. & Schroit, A. J. Bidirectional transbilayer movement of phospholipid analogs in human red blood cells. Evidence for an ATP-dependent and protein-mediated process. J. Biol. Chem. 267, 19412–19417 (1992).
Diaz, C. & Schroit, A. J. Role of translocases in the generation of phosphatidylserine asymmetry. J. Membr. Biol. 151, 1–9 (1996).
Bevers, E. M., Comfurius, P., van Rijn, J. L., Hemker, H. C. & Zwaal, R. F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 122, 429–436 (1982).
Rosing, J., Speijer, H. & Zwaal, R. F. Prothrombin activation on phospholipid membranes with positive electrostatic potential. Biochemistry 27, 8–11 (1988).
van Dieijen, G., Tans, G., Rosing, J. & Hemker, H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J. Biol. Chem. 256, 3433–3442 (1981).
Zwaal, R. F. & Bevers, E. M. Platelet phospholipid asymmetry and its significance in hemostasis. Subcell. Biochem. 9, 299–334 (1983).
Schroit, A. J., Tanaka, Y., Madsen, J. & Fidler, I. J. The recognition of red blood cells by macrophages: role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol. Cell 51, 227–238 (1984).
Beleznay, Z., Zachowski, A., Devaux, P. F., Navazo, M. P. & Ott, P. ATP-dependent aminophospholipid translocation in erythrocyte vesicles: stoichiometry of transport. Biochemistry 32, 3146–3152 (1993).
Zwaal, R. F., Comfurius, P. & Bevers, E. M. Mechanism and function of changes in membrane-phospholipid asymmetry in platelets and erythrocytes. Biochem. Soc. Trans. 21, 248–253 (1993).
Horstman, L. L., Jy, W., Jimenez, J. J., Bidot, C. & Ahn, Y. S. New horizons in the analysis of circulating cell-derived microparticles. Keio J. Med. 53, 210–230 (2004).
Jimenez, J. J. et al. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br. J. Haematol. 123, 896–902 (2003).
Moskovich, O. & Fishelson, Z. Live cell imaging of outward and inward vesiculation induced by the complement c5b-9 complex. J. Biol. Chem. 282, 29977–29986 (2007).
Del Conde, I., Shrimpton, C. N., Thiagarajan, P. & Lopez, J. A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604–1611 (2005).
Miguet, L. et al. Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics 6, 153–171 (2006).
Peterson, D. B. et al. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics 8, 2430–2446 (2008).
Reich, C. F. 3rd & Pisetsky, D. S. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp. Cell Res. 315, 760–768 (2009).
Hasselmann, D. O., Rappl, G., Tilgen, W. & Reinhold, U. Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin. Chem. 47, 1488–1489 (2001).
Dachary-Prigent, J. et al. Aminophospholipid exposure, microvesiculation and abnormal protein tyrosine phosphorylation in the platelets of a patient with Scott syndrome: a study using physiologic agonists and local anaesthetics. Br. J. Haematol. 99, 959–967 (1997).
Toti, F., Satta, N., Fressinaud, E., Meyer, D. & Freyssinet, J. M. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood 87, 1409–1415 (1996).
Zwaal, R. F., Comfurius, P. & Bevers, E. M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta 1636, 119–128 (2004).
Hrachovinova, I. et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat. Med. 9, 1020–1025 (2003).
Proulle, V. et al. Circulating microparticles are elevated in haemophiliacs and non-haemophilic individuals aged <18 years. Br. J. Haematol. 131, 487–489 (2005).
Lazarus, A. H. et al. Comparison of platelet immunity in patients with SLE and with ITP. Transfus. Sci. 22, 19–27 (2000).
Warren, B. A. & Vales, O. The release of vesicles from platelets following adhesion to vessel walls in vitro. Br. J. Exp. Pathol. 53, 206–215 (1972).
Michelson, A. D., Rajasekhar, D., Bednarek, F. J. & Barnard, M. R. Platelet and platelet-derived microparticle surface factor V/Va binding in whole blood: differences between neonates and adults. Thromb. Haemost. 84, 689–694 (2000).
Muller, I. et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 17, 476–478 (2003).
Scholz, T., Temmler, U., Krause, S., Heptinstall, S. & Losche, W. Transfer of tissue factor from platelets to monocytes: role of platelet-derived microvesicles and CD62P. Thromb. Haemost. 88, 1033–1038 (2002).
Siddiqui, F. A., Desai, H., Amirkhosravi, A., Amaya, M. & Francis, J. L. The presence and release of tissue factor from human platelets. Platelets 13, 247–253 (2002).
Zillmann, A. et al. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem. Biophys. Res. Commun. 281, 603–609 (2001).
Jy, W. et al. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. J. Thromb. Haemost. 3, 1301–1308 (2005).
Dignat-George, F. et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb. Haemost. 91, 667–673 (2004).
Jy, W. et al. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb. Res. 121, 319–325 (2007).
Nagahama, M. et al. Significance of anti-oxidized LDL antibody and monocyte-derived microparticles in anti-phospholipid antibody syndrome. Autoimmunity 36, 125–131 (2003).
Pereira, J. et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb. Haemost. 95, 94–99 (2006).
Nomura, S. et al. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 158, 277–287 (2001).
Joseph, J. E., Harrison, P., Mackie, I. J., Isenberg, D. A. & Machin, S. J. Increased circulating platelet–leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 115, 451–459 (2001).
Nagahama, M. et al. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 33, 85–94 (2001).
Knijff-Dutmer, E. A., Koerts, J., Nieuwland, R., Kalsbeek-Batenburg, E. M. & van de Laar, M. A. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 46, 1498–1503 (2002).
Barry, O. P., Pratico, D., Lawson, J. A. & FitzGerald, G. A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 99, 2118–2127 (1997).
Barry, O. P., Pratico, D., Savani, R. C. & FitzGerald, G. A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Invest. 102, 136–144 (1998).
Pfister, S. L. Role of platelet microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension 43, 428–433 (2004).
Brodsky, S. V., Zhang, F., Nasjletti, A. & Goligorsky, M. S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol. 286, H1910–H1915 (2004).
Tesse, A. et al. Upregulation of proinflammatory proteins through NF-κB pathway by shed membrane microparticles results in vascular hyporeactivity. Arterioscler. Thromb. Vasc. Biol. 25, 2522–2527 (2005).
Tesse, A. et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents microparticle-induced vascular hyporeactivity through the regulation of proinflammatory proteins. J. Pharmacol. Exp. Ther. 324, 539–547 (2008).
Brill, A., Dashevsky, O., Rivo, J., Gozal, Y. & Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 67, 30–38 (2005).
Ettelaie, C., Su, S., Li, C. & Collier, M. E. Tissue factor-containing microparticles released from mesangial cells in response to high glucose and AGE induce tube formation in microvascular cells. Microvasc. Res. 76, 152–160 (2008).
Soleti, R. et al. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 30, 580–588 (2009).
Brogan, P. A. & Dillon, M. J. Endothelial microparticles and the diagnosis of the vasculitides. Intern. Med. 43, 1115–1119 (2004).
Daniel, L. et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 69, 1416–1423 (2006).
Erdbruegger, U. et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 47, 1820–1825 (2008).
Bakouboula, B. et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 177, 536–543 (2008).
Amabile, N. et al. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 177, 1268–1275 (2008).
Guiducci, S. et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 58, 2845–2853 (2008).
Nomura, S., Inami, N., Ozaki, Y., Kagawa, H. & Fukuhara, S. Significance of microparticles in progressive systemic sclerosis with interstitial pneumonia. Platelets 19, 192–198 (2008).
Beyer, C., Schett, G., Gay, S., Distler, O. & Distler, J. H. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res. Ther. 11, 220 (2009).
Gasser, O. et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 285, 243–257 (2003).
Hess, C., Sadallah, S., Hefti, A., Landmann, R. & Schifferli, J. A. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 163, 4564–4573 (1999).
Nauta, A. J. et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 32, 1726–1736 (2002).
Gasser, O. & Schifferli, J. A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104, 2543–2548 (2004).
MacKenzie, A. et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 15, 825–835 (2001).
Mesri, M. & Altieri, D. C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 274, 23111–23118 (1999).
Mack, M. et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6, 769–775 (2000).
Rozmyslowicz, T. et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 17, 33–42 (2003).
Forlow, S. B., McEver, R. P. & Nollert, M. U. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood 95, 1317–1323 (2000).
Baj-Krzyworzeka, M. et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol. 30, 450–459 (2002).
Billy, D., Speijer, H., Zwaal, R. F., Hack, E. C. & Hermens, W. T. Anticoagulant and membrane-degrading effects of secretory (non-pancreatic) phospholipase A2 are inhibited in plasma. Thromb. Haemost. 87, 978–984 (2002).
Fourcade, O. et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 80, 919–927 (1995).
Jungel, A. et al. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56, 3564–3574 (2007).
Tabibzadeh, S. S., Kong, Q. F. & Kapur, S. Passive acquisition of leukocyte proteins is associated with changes in phosphorylation of cellular proteins and cell-cell adhesion properties. Am. J. Pathol. 145, 930–940 (1994).
Distler, J. H. et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 10, 731–741 (2005).
Jodo, S. et al. Apoptosis-inducing membrane vesicles. A novel agent with unique properties. J. Biol. Chem. 276, 39938–39944 (2001).
Albanese, J. et al. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood 91, 3862–3874 (1998).
Abrahams, V. M., Straszewski-Chavez, S. L., Guller, S. & Mor, G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol. Hum. Reprod. 10, 55–63 (2004).
Taylor, D. D. & Gercel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 92, 305–311 (2005).
Whitecar, P. W., Boggess, K. A., McMahon, M. J., Thorp, J. M., Jr & Taylor, D. D. Altered expression of TCR-CD3zeta induced by sera from women with preeclampsia. Am. J. Obstet. Gynecol. 185, 812–818 (2001).
Amirkhosravi, A. et al. Platelet microparticles carry CD40 ligand and up-regulate TF and VEGF in endothelial and melanoma cells: possible role in angiogenesis and metastasis. J. Thromb. Haemost. 1 (Suppl. 1), P0822 (2003).
Taraboletti, G. et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160, 673–680 (2002).
Ginestra, A. et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 18, 3433–3437 (1998).
Berckmans, R. J. et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. Ther. 7, R536–R544 (2005).
Berckmans, R. J. et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 46, 2857–2866 (2002).
Biro, E. et al. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann. Rheum. Dis. 66, 1085–1092 (2007).
Messer, L. et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res. Ther. 11, R40 (2009).
Distler, J. H. et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl Acad. Sci. USA 102, 2892–2897 (2005).
Combes, V. et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 104, 93–102 (1999).
Umekita, K. et al. Leukocytapheresis (LCAP) decreases the level of platelet-derived microparticles (MPs) and increases the level of granulocytes-derived MPs: a possible connection with the effect of LCAP on rheumatoid arthritis. Mod. Rheumatol. 19, 265–272 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Beyer, C., Pisetsky, D. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol 6, 21–29 (2010). https://doi.org/10.1038/nrrheum.2009.229
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.229
This article is cited by
-
The role of extracellular vesicles in COPD and potential clinical value
Respiratory Research (2024)
-
Microparticles: potential new contributors to the pathogenesis of systemic sclerosis?
Advances in Rheumatology (2023)
-
Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment
Stem Cell Research & Therapy (2022)
-
Extracellular Vesicle-Mediated Vascular Cell Communications in Hypertension: Mechanism Insights and Therapeutic Potential of ncRNAs
Cardiovascular Drugs and Therapy (2022)
-
Characterization of hyaluronan-coated extracellular vesicles in synovial fluid of patients with osteoarthritis and rheumatoid arthritis
BMC Musculoskeletal Disorders (2021)