Key Points
-
The ubiquitin proteasome system (UPS) controls protein concentrations in eukaryotic cells by setting protein degradation rates. Proteins are targeted to the proteasome by covalently attached ubiquitin tags; the proteasome recognizes these tags, unfolds the protein and translocates it in an ATP-dependent process into a proteolytic chamber, where it is hydrolysed into small peptides.
-
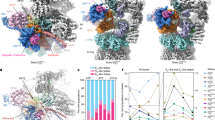
The 26S proteasome consists of ∼33 different subunits that form a cylindrical particle of a molecular weight of ∼2.5 MDa. In the past 2 years, the structure of the entire proteasome has been revealed at near-atomic resolution in a series of breakthrough studies.
-
The structures show that the biochemical activities of the proteasome are arranged in the order in which they act on a substrate: the two ubiquitin receptors are located near to the ends of the particle, followed by a protease that cleaves the ubiquitin tag from substrates, the ATPase motor that translocates substrates into the proteasome and finally the proteolytic sites in a chamber at the centre of the particle.
-
The arrangement of ubiquitin receptors, substrate channel and ATPase motor in the proteasome seems to form a versatile platform that allows substrates to be recognized in multiple ways and to be fed into the degradation machinery.
-
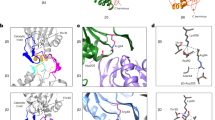
Proteasome structures in the presence and absence of substrate and ATP analogues show that the proteasome adopts distinct conformations under the different conditions, and the structures give insights into how it might be allosterically regulated. The binding of a substrate or ATP analogue switches the proteasome from a presumably inactive structure, in which parts of the substrate translocation channel are misaligned, into an active structure with an unobstructed channel.
-
Substrate binding affects the structure of the ATPase ring that forms the translocation motor of the proteasome and switches it from a spiral to a more planar ring. The rearrangements might represent resting and active states of the proteasome and suggest several models by which changes of the ATPase subunits could move substrate through the translocation channel.
Abstract
The ubiquitin proteasome system (UPS) is the main ATP-dependent protein degradation pathway in the cytosol and nucleus of eukaryotic cells. At its centre is the 26S proteasome, which degrades regulatory proteins and misfolded or damaged proteins. In a major breakthrough, several groups have determined high-resolution structures of the entire 26S proteasome particle in different nucleotide conditions and with and without substrate using cryo-electron microscopy combined with other techniques. These structures provide some surprising insights into the functional mechanism of the proteasome and will give invaluable guidance for genetic and biochemical studies of this key regulatory system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Belle, A., Tanay, A., Bitincka, L., Shamir, R. & O'Shea, E.K. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci USA 103, 13004–13009 (2006). The first study of system-wide protein turnover. Shows among other things that protein degradation rates vary over two orders of magnitude in yeast and are thus an important determinant of cellular protein concentrations.
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Varshavsky, A. The ubiquitin system. Trends Biochem. Sci. 22, 383–387 (1997).
Coux, O., Tanaka, K. & Goldberg, A. L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847 (1996).
Hochstrasser, M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30, 405–439 (1996).
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009). A comprehensive survey of proteasome function and its mechanism.
Verma, R., McDonald, H., Yates, J. R. & Deshaies, R. J. Selective degradation of ubiquitinated Sic1 by purified 26S proteasome yields active S phase cyclin–Cdk. Mol. Cell 8, 439–448 (2001). An elegant example of in vitro biochemical analysis of proteasome degradation. Demonstrates that the proteasome can extract a single polypeptide chain out of a protein complex, including highly proteasome-sensitive cyclin.
Nishiyama, A. et al. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 14, 2344–2357 (2000).
Johnson, E.S., Gonda, D.K. & Varshavsky, A. Cis–trans recognition and subunit-specific degradation of short-lived proteins. Nature 346, 287–291 (1990).
Hochstrasser, M. & Varshavsky, A. In vivo degradation of a transcriptional regulator: the yeast α2 repressor. Cell 61, 697–708 (1990).
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012). Authoritative review of polyubiquitin chains as they are found in cells.
Hicke, L. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol. 2, 195–201 (2001).
Jentsch, S. The ubiquitin-conjugation system. Annu. Rev. Genet. 26, 179–207 (1992).
MacGurn, J. A., Hsu, P. C. & Emr, S. D. Ubiquitin and membrane protein turnover: from cradle to grave. Annu. Rev. Biochem. 81, 231–259 (2012).
Mukhopadhyay, D. & Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205 (2007).
Vucic, D., Dixit, V. M. & Wertz, I. E. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nature Rev. Mol. Cell Biol. 12, 439–452 (2011).
Goldknopf, I. L., French, M. F., Musso, R. & Busch, H. Presence of protein A24 in rat liver nucleosomes. Proc. Natl Acad. Sci. USA 74, 5492–5495 (1977).
Levinger, L. & Varshavsky, A. High-resolution fractionation of nucleosomes: minor particles, “whiskers,” and separation of mononucleosomes containing and lacking A24 semihistone. Proc. Natl Acad. Sci. USA 77, 3244–3248 (1980).
Trempe, J. F. Reading the ubiquitin postal code. Curr. Opin. Struct. Biol. 21, 792–801 (2011).
Prakash, S., Tian, L., Ratliff, K. S., Lehotzky, R. E. & Matouschek, A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nature Struct. Mol. Biol. 11, 830–837 (2004). In vitro study of proteasome degradation. Describes a second component of the proteasome degradation signal in the form of an intrinsically disordered region in the substrate protein that serves as the proteasome initiation site.
Lee, C., Schwartz, M. P., Prakash, S., Iwakura, M. & Matouschek, A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol. Cell 7, 627–637 (2001). Shows that the proteasome degrades proteins sequentially by running along their polypeptide chain and that different protein structures vary substantially in their susceptibility to proteasomal unfolding and digestion.
Nussbaum, A. K. et al. Cleavage motifs of the yeast 20S proteasome β subunits deduced from digests of enolase 1. Proc. Natl Acad. Sci. USA 95, 12504–12509 (1998).
Kisselev, A. F., Akopian, T. N., Woo, K. M. & Goldberg, A. L. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J. Biol. Chem. 274, 3363–3371 (1999).
Verma, R. et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298, 611–615 (2002).
Yao, T. & Cohen, R. E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419, 403–407 (2002). References 24 and 25 describe the deubiquitylation acitivty of Rpn11 as an ATP-dependent and essential step in proteasomal protein degradation.
Prakash, S., Inobe, T., Hatch, A. J. & Matouschek, A. Substrate selection by the proteasome during degradation of protein complexes. Nature Chem. Biol. 5, 29–36 (2009).
Palombella, V. J., Rando, O. J., Goldberg, A. L. & Maniatis, T. The ubiquitin–proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78, 773–785 (1994). The first study to describe an alternative proteasome function that post-translationally remodels proteins by degrading them only partially.
Aza-Blanc, P., Ramirez-Weber, F. A., Laget, M. P., Schwartz, C. & Kornberg, T. B. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89, 1043–1053 (1997).
Wilson, M. D. et al. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 154, 983–995 (2013).
Hoppe, T. et al. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102, 577–586 (2000).
Tian, L., Holmgren, R. A. & Matouschek, A. A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-κB. Nature Struct. Mol. Biol. 12, 1045–1053 (2005). References 28–31 describe additional examples of partial degradation by the proteasome as described in reference 27 and begin to uncover the mechanism of this process.
Lowe, J. et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 Å resolution. Science 268, 533–539 (1995).
Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 (1997). The first atomic-resolution structure of a eukaryotic 20S core particle that shows the locations of all 28 subunits and describes the proteolytic cleavage preferences and mechanism of the proteolytic active sites.
Unno, M. et al. The structure of the mammalian 20S proteasome at 2.75 Å resolution. Structure 10, 609–618 (2002).
Groll, M. et al. A gated channel into the proteasome core particle. Nature Struct. Biol. 7, 1062–1067 (2000).
Borissenko, L. & Groll, M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem. Rev. 107, 687–717 (2007).
Baumeister, W., Walz, J., Zuhl, F. & Seemuller, E. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92, 367–380 (1998).
Whitby, F. G. et al. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature 408, 115–120 (2000). Atomic-resolution structure of an 11S regulator from trypanosomes with a 20S core particle from yeast demonstrating how the 11S regulator gates the 20S core particle.
Stadtmueller, B. M. & Hill, C. P. Proteasome activators. Mol. Cell 41, 8–19 (2011).
Sadre-Bazzaz, K., Whitby, F. G., Robinson, H., Formosa, T. & Hill, C. P. Structure of a Blm10 complex reveals common mechanisms for proteasome binding and gate opening. Mol. Cell 37, 728–735 (2010).
Forster, A., Masters, E. I., Whitby, F. G., Robinson, H. & Hill, C. P. The 1.9 Å structure of a proteasome–11S activator complex and implications for proteasome-PAN/PA700 interactions. Mol. Cell 18, 589–599 (2005).
Kish-Trier, E. & Hill, C. P. Structural biology of the proteasome. Annu. Rev. Biophys. 42, 29–49 (2013).
Cascio, P., Call, M., Petre, B. M., Walz, T. & Goldberg, A. L. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J. 21, 2636–2645 (2002).
Tanahashi, N. et al. Hybrid proteasomes. Induction by interferon-γ and contribution to ATP-dependent proteolysis. J. Biol. Chem. 275, 14336–14345 (2000).
Shibatani, T. et al. Global organization and function of mammalian cytosolic proteasome pools: implications for PA28 and 19S regulatory complexes. Mol. Biol. Cell 17, 4962–4971 (2006).
Schmidt, M. et al. The HEAT repeat protein Blm10 regulates the yeast proteasome by capping the core particle. Nature Struct. Mol. Biol. 12, 294–303 (2005).
Barthelme, D. & Sauer, R. T. Identification of the Cdc48*20S proteasome as an ancient AAA+ proteolytic machine. Science 337, 843–846 (2012). Shows that the AAA+ chaperone CDC48 might function as a novel proteasome activator cap.
Barthelme, D. & Sauer, R. T. Bipartite determinants mediate an evolutionarily conserved interaction between Cdc48 and the 20S peptidase. Proc. Natl Acad. Sci. USA 110, 3327–3332 (2013).
Zhang, Z. et al. Identification of an activation region in the proteasome activator REGα. Proc. Natl Acad. Sci. USA 95, 2807–2811 (1998).
Smith, D. M. et al. Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's α ring opens the gate for substrate entry. Mol. Cell 27, 731–744 (2007).
Gillette, T. G., Kumar, B., Thompson, D., Slaughter, C. A. & DeMartino, G. N. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J. Biol. Chem. 283, 31813–31822 (2008).
Rabl, J. et al. Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol. Cell 30, 360–368 (2008).
Glickman, M. H. et al. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94, 615–623 (1998). Defines the lid and base subcomplexes of the proteasome and identifies the subunits that belong to each subcomplex.
Chen, L. & Madura, K. Rad23 promotes the targeting of proteolytic substrates to the proteasome. Mol. Cell. Biol. 22, 4902–4913 (2002).
Verma, R., Oania, R., Graumann, J. & Deshaies, R. J. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin–proteasome system. Cell 118, 99–110 (2004).
Elsasser, S., Chandler-Militello, D., Muller, B., Hanna, J. & Finley, D. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J. Biol. Chem. 279, 26817–26822 (2004).
Elsasser, S. & Finley, D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nature Cell Biol. 7, 742–749 (2005).
Kim, I., Mi, K. & Rao, H. Multiple interactions of Rad23 suggest a mechanism for ubiquitylated substrate delivery important in proteolysis. Mol. Biol. Cell 15, 3357–3365 (2004).
Kaplun, L. et al. The DNA damage-inducible UbL-UbA protein Ddi1 participates in Mec1–mediated degradation of Ho endonuclease. Mol. Cell. Biol. 25, 5355–5362 (2005).
Funakoshi, M., Sasaki, T., Nishimoto, T. & Kobayashi, H. Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc. Natl Acad. Sci. USA 99, 745–750 (2002).
Wilkinson, C. R. et al. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nature Cell Biol. 3, 939–943 (2001).
Saeki, Y., Saitoh, A., Toh-e, A. & Yokosawa, H. Ubiquitin-like proteins and Rpn10 play cooperative roles in ubiquitin-dependent proteolysis. Biochem. Biophys. Res. Commun. 293, 986–992 (2002).
Hanna, J. et al. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 127, 99–111 (2006).
Crosas, B. et al. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127, 1401–1413 (2006). Good example of how deubiquitylation and ubiqutin ligase activities on the proteasome cooperate to edit ubiquitin chains on the proteasome.
Lam, Y. A., Xu, W., DeMartino, G. N. & Cohen, R. E. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385, 737–740 (1997).
Lee, M. J., Lee, B. H., Hanna, J., King, R. W. & Finley, D. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol. Cell. Proteomics 10, R110.003871 (2011).
Xie, Y. & Varshavsky, A. Physical association of ubiquitin ligases and the 26S proteasome. Proc. Natl Acad. Sci. USA 97, 2497–2502 (2000).
Guterman, A. & Glickman, M. H. Complementary roles for Rpn11 and Ubp6 in deubiquitination and proteolysis by the proteasome. J. Biol. Chem. 279, 1729–1738 (2004)
Borodovsky, A. et al. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 20, 5187–5196 (2001).
Walz, J. et al. 26S proteasome structure revealed by three-dimensional electron microscopy. J. Struct. Biol. 121, 19–29 (1998). One of the first medium-resolution structures of the entire 26S proteasome, revealed using electron microscopy.
da Fonseca, P. C. & Morris E. P. Structure of the human 26S proteasome: subunit radial displacements open the gate into the proteolytic core. J. Biol. Chem. 283, 23305–23314 (2008).
Bohn, S. et al. Structure of the 26S proteasome from Schizosaccharomyces pombe at subnanometer resolution. Proc. Natl Acad. Sci. USA 107, 20992–20997 (2010).
Zhang, F. et al. Structural insights into the regulatory particle of the proteasome from Methanocaldococcus jannaschii. Mol. Cell 34, 473–484 (2009).
Zhang, F. et al. Mechanism of substrate unfolding and translocation by the regulatory particle of the proteasome from Methanocaldococcus jannaschii. Mol. Cell 34, 485–496 (2009).
Djuranovic, S. et al. Structure and activity of the N-terminal substrate recognition domains in proteasomal ATPases. Mol. Cell 34, 580–590 (2009).
Sakata, E. et al. Localization of the proteasomal ubiquitin receptors Rpn10 and Rpn13 by electron cryomicroscopy. Proc. Natl Acad. Sci. USA 109, 1479–1484 (2012).
Lander, G. C. et al. Complete subunit architecture of the proteasome regulatory particle. Nature 482, 186–191 (2012).
Lasker, K. et al. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc. Natl Acad. Sci. USA 109, 1380–1387 (2012).
da Fonseca, P. C., He, J. & Morris, E. P. Molecular model of the human 26S proteasome. Mol. Cell 46, 54–66 (2012).
Beck, F. et al. Near-atomic resolution structural model of the yeast 26S proteasome. Proc. Natl Acad. Sci. USA 109, 14870–14875 (2012).
Matyskiela, M. E., Lander, G. C. & Martin, A. Conformational switching of the 26S proteasome enables substrate degradation. Nature Struct. Mol. Biol. 20, 781–788 (2013).
Sledz, P. et al. Structure of the 26S proteasome with ATP-γS bound provides insights into the mechanism of nucleotide-dependent substrate translocation. Proc. Natl Acad. Sci. USA 110, 7264–7269 (2013). References 77–82 describe the recent structures of the 26S proteasome at near-atomic resolution.
Matyskiela, M. E. & Martin, A. Design principles of a universal protein degradation machine. J. Mol. Biol. 425, 199–213 (2012).
Lander, G. C., Martin, A. & Nogales, E. The proteasome under the microscope: the regulatory particle in focus. Curr. Opin. Struct. Biol. 23, 243–251 (2013).
Bohn, S. et al. Localization of the regulatory particle subunit Sem1 in the 26S proteasome. Biochem. Biophys. Res. Commun. 435, 250–254 (2013).
Tomko, R. J. Jr, Funakoshi, M., Schneider, K., Wang, J. & Hochstrasser, M. Heterohexameric ring arrangement of the eukaryotic proteasomal ATPases: implications for proteasome structure and assembly. Mol. Cell 38, 393–403 (2010).
Hanson, P. I. & Whiteheart, S. W. AAA+ proteins: have engine, will work. Nature Rev. Mol. Cell Biol. 6, 519–529 (2005).
Tomko, R. J. Jr & Hochstrasser, M. Molecular architecture and assembly of the eukaryotic proteasome. Annu. Rev. Biochem. 82, 415–445 (2013).
Pathare, G. R. et al. The proteasomal subunit Rpn6 is a molecular clamp holding the core and regulatory subcomplexes together. Proc. Natl Acad. Sci. USA 109, 149–154 (2012).
Santamaria, P. G., Finley, D., Ballesta, J. P. & Remacha, M. Rpn6p, a proteasome subunit from Saccharomyces cerevisiae, is essential for the assembly and activity of the 26 S proteasome. J. Biol. Chem. 278, 6687–6695 (2003).
Isono, E., Saito, N., Kamata, N., Saeki, Y. & Toh, E. A. Functional analysis of Rpn6p, a lid component of the 26 S proteasome, using temperature-sensitive rpn6 mutants of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 280, 6537–6547 (2005).
Kim, Y. C., Li, X., Thompson, D. & Demartino, G. N. ATP binding by proteasomal ATPases regulates cellular assembly and substrate-induced functions of the 26 S proteasome. J. Biol. Chem. 288, 3334–3345 (2013).
Peth, A., Besche, H. C. & Goldberg, A. L. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol. Cell 36, 794–804 (2009).
Li, X. & Demartino, G. N. Variably modulated gating of the 26S proteasome by ATP and polyubiquitin. Biochem. J. 421, 397–404 (2009).
Bech-Otschir, D. et al. Polyubiquitin substrates allosterically activate their own degradation by the 26S proteasome. Nature Struct. Mol. Biol. 16, 219–225 (2009). Describes allosteric activation of proteasomal degradation by polyubiquitylated substrates.
Gur, E. & Sauer, R. T. Degrons in protein substrates program the speed and operating efficiency of the AAA+ Lon proteolytic machine. Proc. Natl Acad. Sci. USA 106, 18503–18508 (2009).
Sen, M. et al. The ClpXP protease unfolds substrates using a constant rate of pulling but different gears. Cell 155, 636–646 (2013). References 93–97 discuss allosteric effects in the proteasome and bacterial analogues.
Smith, D. M., Fraga, H., Reis, C., Kafri, G. & Goldberg, A. L. ATP binds to proteasomal ATPases in pairs with distinct functional effects, implying an ordered reaction cycle. Cell 144, 526–538 (2011). Shows that the archaeal proteasome ATPases and, by extension, the eukaryotic proteasomal ATPases hydrolyse ATP in a coordinated manner.
Thrower, J. S., Hoffman, L., Rechsteiner, M. & Pickart, C. M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000). Biochemical analysis of ubiquitin chain recognition by the proteasome that establishes the tetraubiquitin chain as a key component of the proteasome degron.
Ravid, T. & Hochstrasser, M. Diversity of degradation signals in the ubiquitin–proteasome system. Nature Rev. Mol. Cell Biol. 9, 679–690 (2008).
Dimova, N. V. et al. APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1. Nature Cell Biol. 14, 168–176 (2012).
Husnjak, K. et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 (2008). Shows that Rpn13 serves as an ubiquitin receptor on the proteasome and investigates how the different ubiquitin receptors contribute to protein degradation in vivo.
Deveraux, Q., Ustrell, V., Pickart, C. & Rechsteiner, M. A. 26 S protease subunit that binds ubiquitin conjugates. J. Biol. Chem. 269, 7059–7061 (1994).
Schreiner, P. et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature 453, 548–552 (2008).
Wang, Q., Young, P. & Walters, K. J. Structure of S5a bound to monoubiquitin provides a model for polyubiquitin recognition. J. Mol. Biol. 348, 727–739 (2005).
Zhang, D. et al. Together, Rpn10 and Dsk2 can serve as a polyubiquitin chain-length sensor. Mol. Cell 36, 1018–1033 (2009).
Varadan, R., Walker, O., Pickart, C. & Fushman, D. Structural properties of polyubiquitin chains in solution. J. Mol. Biol. 324, 637–647 (2002).
Eddins, M. J., Varadan, R., Fushman, D., Pickart, C. M. & Wolberger, C. Crystal structure and solution NMR studies of Lys48-linked tetraubiquitin at neutral pH. J. Mol. Biol. 367, 204–211 (2007).
Ryabov, Y. & Fushman, D. Interdomain mobility in di-ubiquitin revealed by NMR. Proteins 63, 787–796 (2006).
Zhang, N. et al. Structure of the s5a:k48-linked diubiquitin complex and its interactions with rpn13. Mol. Cell 35, 280–290 (2009).
Tenno, T. et al. Structural basis for distinct roles of Lys63- and Lys48-linked polyubiquitin chains. Genes Cells 9, 865–875 (2004).
Datta, A. B., Hura, G. L. & Wolberger, C. The structure and conformation of Lys63-linked tetraubiquitin. J. Mol. Biol. 392, 1117–1124 (2009).
Zhao, M., Zhang, N. Y., Zurawel, A., Hansen, K. C. & Liu, C. W. Degradation of some polyubiquitinated proteins requires an intrinsic proteasomal binding element in the substrates. J. Biol. Chem. 285, 4771–4780 (2010).
Takeuchi, J., Chen, H. & Coffino, P. Proteasome substrate degradation requires association plus extended peptide. EMBO J. 26, 123–131 (2007).
Verhoef, L. G. et al. Minimal length requirement for proteasomal degradation of ubiquitin-dependent substrates. FASEB J. 23, 123–133 (2009).
Heinen, C., Acs, K., Hoogstraten, D. & Dantuma, N. P. C-terminal UBA domains protect ubiquitin receptors by preventing initiation of protein degradation. Nature Commun. 2, 191 (2011).
Fishbain, S., Prakash, S., Herrig, A., Elsasser, S. & Matouschek, A. Rad23 escapes degradation because it lacks a proteasome initiation region. Nature Commun. 2, 192 (2011).
Inobe, T., Fishbain, S., Prakash, S. & Matouschek, A. Defining the geometry of the two-component proteasome degron. Nature Chem. Biol. 7, 161–167 (2011).
Deshaies, R. J. & Joazeiro, C. A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 (2009). Describes the biology and mechanism of action of RING finger ubiquitin ligases.
Elsasser, S. et al. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nature Cell Biol. 4, 725–730 (2002).
Saeki, Y., Sone, T., Toh-e, A. & Yokosawa, H. Identification of ubiquitin-like protein-binding subunits of the 26S proteasome. Biochem. Biophys. Res. Commun. 296, 813–819 (2002).
Walters, K. J., Lech, P. J., Goh, A. M., Wang, Q. & Howley, P. M. DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proc. Natl Acad. Sci. USA 100, 12694–12699 (2003).
Hoyt, M. A. & Coffino, P. Ubiquitin-free routes into the proteasome. Cell. Mol. Life Sci. 61, 1596–1600 (2004).
Lee, B. H. et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 (2010). Describes small-molecule inhibitors of the deubiquitylating activity on the proteasome as therapeutic strategies to treat neurodegenerative diseases.
Aviram, S. & Kornitzer, D. The ubiquitin ligase Hul5 promotes proteasomal processivity. Mol. Cell. Biol. 30, 985–994 (2010).
Piwko, W. & Jentsch, S. Proteasome-mediated protein processing by bidirectional degradation initiated from an internal site. Nature Struct. Mol. Biol. 13, 691–697 (2006).
Lin, L. & Ghosh, S. A glycine-rich region in NF-κB p105 functions as a processing signal for the generation of the p50 subunit. Mol. Cell. Biol. 16, 2248–2254 (1996).
Zhang, M. & Coffino, P. Repeat sequence of Epstein–Barr virus-encoded nuclear antigen 1 protein interrupts proteasome substrate processing. J. Biol. Chem. 279, 8635–8641 (2004).
Erzberger, J. P. & Berger, J. M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006).
Sauer, R. T. & Baker, T. A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 (2011). A recent comprehensive review of AAA+ proteases and their mechanism of substrate recognition and ATP-dependent degradation.
Smith, D. M., Benaroudj, N. & Goldberg, A. Proteasomes and their associated ATPases: a destructive combination. J. Struct. Biol. 156, 72–83 (2006).
Wang, J. et al. Crystal structures of the HslVU peptidase–ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9, 177–184 (2001). One of the first papers to identify the aromatic-hydrophobic-Gly motif loop as a key component in the ATPase motor of AAA+ proteases.
Hinnerwisch, J., Fenton, W. A., Furtak, K. J., Farr, G. W. & Horwich, A. L. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell 121, 1029–1041 (2005).
Martin, A., Baker, T. A. & Sauer, R. T. Diverse pore loops of the AAA+ ClpX machine mediate unassisted and adaptor-dependent recognition of ssrA-tagged substrates. Mol. Cell 29, 441–450 (2008).
Martin, A., Baker, T. A. & Sauer, R. T. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nature Struct. Mol. Biol. 15, 1147–1151 (2008).
Wang, J. et al. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure 9, 1107–1116 (2001).
Yamada-Inagawa, T., Okuno, T., Karata, K., Yamanaka, K. & Ogura, T. Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J. Biol. Chem. 278, 50182–50187 (2003).
Park, E. et al. Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J. Biol. Chem. 280, 22892–22898 (2005).
Koga, N., Kameda, T., Okazaki, K. & Takada, S. Paddling mechanism for the substrate translocation by AAA+ motor revealed by multiscale molecular simulations. Proc. Natl Acad. Sci. USA 106, 18237–18242 (2009).
Aubin-Tam, M. E., Olivares, A. O., Sauer, R. T., Baker, T. A. & Lang, M. J. Single-molecule protein unfolding and translocation by an ATP-fueled proteolytic machine. Cell 145, 257–267 (2011).
Maillard, R. A. et al. ClpX(P) generates mechanical force to unfold and translocate its protein substrates. Cell 145, 459–469 (2011). References 140 and 141 are the first demonstrations of force generation by AAA+ proteases using single-molecule methods.
Glynn, S. E., Martin, A., Nager, A. R., Baker, T. A. & Sauer, R. T. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell 139, 744–756 (2009). Atomic-resolution structures of ClpX hexamers that suggest how the six subunits interact with one another, using ATP hydrolysis to drive the motor of the ClpXP protease.
Glynn, S. E., Nager, A. R., Baker, T. A. & Sauer, R. T. Dynamic and static components power unfolding in topologically closed rings of a AAA+ proteolytic machine. Nature Struct. Mol. Biol. 19, 616–622 (2012).
Hersch, G. L., Burton, R. E., Bolon, D. N., Baker, T. A. & Sauer, R. T. Asymmetric interactions of ATP with the AAA+ ClpX6 unfoldase: allosteric control of a protein machine. Cell 121, 1017–1027 (2005).
Horwitz, A. A. et al. ATP-induced structural transitions in PAN, the proteasome-regulatory ATPase complex in Archaea. J. Biol. Chem. 282, 22921–22929 (2007).
Yakamavich, J. A., Baker, T. A. & Sauer, R. T. Asymmetric nucleotide transactions of the HslUV protease. J. Mol. Biol. 380, 946–957 (2008).
Stinson, B. M. et al. Nucleotide binding and conformational switching in the hexameric ring of a AAA+ machine. Cell 153, 628–639 (2013).
Augustin, S. et al. An intersubunit signaling network coordinates ATP hydrolysis by m-AAA proteases. Mol. Cell 35, 574–585 (2009).
Martin, A., Baker, T. A. & Sauer, R. T. Rebuilt AAA+ motors reveal operating principles for ATP-fuelled machines. Nature 437, 1115–1120 (2005). Describes the coordination of ATP hydrolysis between subunits of the translocation motor of ClpXP.
Herman, C., Prakash, S., Lu, C. Z., Matouschek, A. & Gross, C. A. Lack of a robust unfoldase activity confers a unique level of substrate specificity to the universal AAA protease FtsH. Mol. Cell 11, 659–669 (2003).
Koodathingal, P. et al. ATP-dependent proteases differ substantially in their ability to unfold globular proteins. J. Biol. Chem. 284, 18674–18684 (2009).
Kohler, A. et al. The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol. Cell 7, 1143–1152 (2001).
Rubin, D. M., Glickman, M. H., Larsen, C. N., Dhruvakumar, S. & Finley, D. Active site mutants in the six regulatory particle ATPases reveal multiple roles for ATP in the proteasome. EMBO J. 17, 4909–4919 (1998). The first of several papers demonstrating that the six ATPases of the eukoryotic proteasome have non-identical roles.
Peth, A., Nathan, J. A. & Goldberg, A. L. The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J. Biol. Chem. 288, 29215–29222 (2013).
Beckwith, R., Estrin, E., Worden, E. J. & Martin, A. Reconstitution of the 26S proteasome reveals functional asymmetries in its AAA+ unfoldase. Nature Struct. Mol. Biol. 20, 1164–1172 (2013).
Erales, J., Hoyt, M. A., Troll, F. & Coffino, P. Functional asymmetries of proteasome translocase pore. J. Biol. Chem. 287, 18535–18543 (2012).
Enemark, E. J. & Joshua-Tor, L. On helicases and other motor proteins. Curr. Opin. Struct. Biol. 18, 243–257 (2008).
Skordalakes, E. & Berger, J. M. Structural insights into RNA-dependent ring closure and ATPase activation by the Rho termination factor. Cell 127, 553–564 (2006).
Costa, A. et al. The structural basis for MCM2-7 helicase activation by GINS and Cdc45. Nature Struct. Mol. Biol. 18, 471–477 (2011).
Enemark, E. J. & Joshua-Tor, L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature 442, 270–275 (2006).
Thomsen, N. D. & Berger, J. M. Running in reverse: the structural basis for translocation polarity in hexameric helicases. Cell 139, 523–534 (2009).
Itsathitphaisarn, O., Wing, R. A., Eliason, W. K., Wang, J. & Steitz, T. A. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell 151, 267–277 (2012).
Chemla, Y. R. et al. Mechanism of force generation of a viral DNA packaging motor. Cell 122, 683–692 (2005).
Aathavan, K. et al. Substrate interactions and promiscuity in a viral DNA packaging motor. Nature 461, 669–673 (2009).
Moffitt, J. R. et al. Intersubunit coordination in a homomeric ring ATPase. Nature 457, 446–450 (2009).
Kessler, B. M. Ubiquitin — omics reveals novel networks and associations with human disease. Curr. Opin. Chem. Biol. 17, 59–65 (2013).
Tai, H. C. & Schuman, E. M. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nature Rev. Neurosci. 9, 826–838 (2008).
Pettersen, E. F. et al. UCSF Chimera — a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pintilie, G. D., Zhang, J., Goddard, T. D., Chiu, W. & Gossard, D. C. Quantitative analysis of cryo-EM density map segmentation by watershed and scale-space filtering, and fitting of structures by alignment to regions. J. Struct. Biol. 170, 427–438 (2010).
Acknowledgements
The authors would like to thank A. Martin and M. Matyskiela for providing segmented electron density maps of their proteasome structures, and members of the Matouschek laboratory, especially J. Renn for critically reading this manuscript. Work in the authors' laboratory is supported by the Gates Foundation, the Welch Foundation and US National Institutes of Health grants R01 GM63004, R01 GM094479, U54 GM105816 and U54 CA143869.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bhattacharyya, S., Yu, H., Mim, C. et al. Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol 15, 122–133 (2014). https://doi.org/10.1038/nrm3741
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3741