Key Points
-
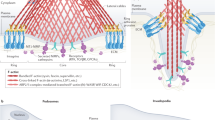
Podosomes and invadopodia are actin-based dynamic protrusions of the plasma membrane. They act as sites of attachment to — and degradation of — the extracellular matrix.
-
These structures contain actin regulators such as cortactin and neural Wiskott–Aldrich syndrome protein (N-WASP), adaptor proteins such as Tyr kinase substrate with four SH3 domains (TKS4) and Tyr kinase substrate with five SH3 domains (TKS5), and several pericellular proteases.
-
Podosomes are found in vascular smooth muscle and endothelial cells, as well as in cells derived from monocyte lineages. Their presence correlates with migratory ability.
-
Invadopodia are found in invasive human cancer cells. In two-dimensional culture, their presence correlates with invasive behaviour. However, in three-dimensional culture and in vivo, invadopodium-associated proteins are also required for cell growth.
-
Podosome-associated proteins have been implicated in human developmental and immune disorders, and dysregulation of podosome formation is associated with atherosclerosis.
-
Small-molecule regulation of podosomes and invadopodia might represent a new therapeutic strategy to treat several diseases.
Abstract
Podosomes and invadopodia are actin-based dynamic protrusions of the plasma membrane of metazoan cells that represent sites of attachment to — and degradation of — the extracellular matrix. The key proteins in these structures include the actin regulators cortactin and neural Wiskott–Aldrich syndrome protein (N-WASP), the adaptor proteins Tyr kinase substrate with four SH3 domains (TKS4) and Tyr kinase substrate with five SH3 domains (TKS5), and the metalloprotease membrane type 1 matrix metalloprotease (MT1MMP; also known as MMP14). Many cell types can produce these structures, including invasive cancer cells, vascular smooth muscle and endothelial cells, and immune cells such as macrophages and dendritic cells. Recently, progress has been made in our understanding of the regulatory and functional aspects of podosome and invadopodium biology and their role in human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
David-Pfeuty, T. & Singer, S. J. Altered distributions of the cytoskeletal proteins vinculin and α-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc. Natl Acad. Sci. USA 77, 6687–6691 (1980).
Tarone, G., Cirillo, D., Giancotti, F. G., Comoglio, P. M. & Marchisio, P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 159, 141–157 (1985).
Chen, W. T., Chen, J. M., Parsons, S. J. & Parsons, J. T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature 316, 156–158 (1985).
Chen, W. T. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 251, 167–185 (1989).
Zambonin-Zallone, A., Teti, A., Carano, A. & Marchisio, P. C. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J. Bone Miner. Res. 3, 517–523 (1988).
Gimona, M., Buccione, R., Courtneidge, S. A. & Linder, S. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 20, 235–241 (2008).
Linder, S. & Kopp, P. Podosomes at a glance. J. Cell Sci. 118, 2079–2082 (2005).
Buccione, R., Caldieri, G. & Ayala, I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 28, 137–149 (2009).
Stylli, S. S., Kaye, A. H. & Lock, P. Invadopodia: at the cutting edge of tumour invasion. J. Clin. Neurosci. 15, 725–737 (2008).
Clark, E. S. & Weaver, A. M. A new role for cortactin in invadopodia: regulation of protease secretion. Eur. J. Cell Biol. 87, 581–590 (2008).
Weaver, A. M. Invadopodia. Curr. Biol. 18, R362–R364 (2008).
Artym, V. V., Matsumoto, K., Mueller, S. C. & Yamada, K. M. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur. J. Cell Biol. 90, 172–180 (2011).
Gawden-Bone, C. et al. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J. Cell Sci. 123, 1427–1437 (2010). This is the first description of highly protrusive podosomes that have the ability to extensively degrade the ECM in a similar manner to invadopodia.
Block, M. R. et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur. J. Cell Biol. 87, 491–506 (2008).
Artym, V. V., Zhang, Y., Seillier-Moiseiwitsch, F., Yamada, K. M. & Mueller, S. C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034–3043 (2006).
Clark, E. S., Whigham, A. S., Yarbrough, W. G. & Weaver, A. M. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227–4235 (2007). This is the first demonstration of the regulated secretion of MMPs at invadopodia.
Linder, S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17, 107–117 (2007).
Ayala, I., Baldassarre, M., Caldieri, G. & Buccione, R. Invadopodia: a guided tour. Eur. J. Cell Biol. 85, 159–164 (2006).
Burgstaller, G. & Gimona, M. Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 288, H3001–H3005 (2005).
Quintavalle, M., Elia, L., Condorelli, G. & Courtneidge, S. A. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J. Cell Biol. 189, 13–22 (2010). This is the first demonstration that miRNAs can control podosome formation, and the first time that podosomes have been visualized in vivo.
Rottiers, P. et al. TGFβ-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J. Cell Sci. 122, 4311–4318 (2009). The first visualization of podosomes in ex vivo cultures of endothelial cells.
Varon, C. et al. Transforming growth factor β induces rosettes of podosomes in primary aortic endothelial cells. Mol. Cell Biol. 26, 3582–3594 (2006).
Petrie, R. J., Doyle, A. D. & Yamada, K. M. Random versus directionally persistent cell migration. Nature Rev. Mol. Cell Biol. 10, 538–549 (2009).
Wolf, K. et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nature Cell Biol. 9, 893–904 (2007).
Albiges-Rizo, C., Destaing, O., Fourcade, B., Planus, E. & Block, M. R. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 122, 3037–3049 (2009).
Collin, O. et al. Self-organized podosomes are dynamic mechanosensors. Curr. Biol. 18, 1288–1294 (2008).
Baldassarre, M. et al. Actin dynamics at sites of extracellular matrix degradation. Eur. J. Cell Biol. 85, 1217–1231 (2006).
Abram, C. L. et al. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 278, 16844–16851 (2003).
Diaz, B. et al. Tks5-dependent, Nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2, ra53 (2009). This paper provides the first demonstration that ROS control the formation of invadopodia and podosomes.
Oikawa, T., Itoh, T. & Takenawa, T. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 182, 157–169 (2008). This paper describes how invadopodia form near focal adhesions at membrane sites containing PtdIns(3,4)P 2.
Stylli, S. S. et al. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J. Cell Sci. 122, 2727–2740 (2009).
Seals, D. F. et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7, 155–165 (2005). This paper shows that TKS5 is essential for invadopodium formation and invasion, and that its expression can promote invadopodium formation in non-invasive cancer cells.
Destaing, O. et al. β1A integrin is a master regulator of invadosome organization and function. Mol. Biol. Cell 21, 4108–4119 (2010). A mechanistic analysis of how integrins regulate invadopodium and podosome formation.
Tatin, F., Varon, C., Genot, E. & Moreau, V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J. Cell Sci. 119, 769–781 (2006).
Billottet, C. et al. Regulatory signals for endothelial podosome formation. Eur. J. Cell Biol. 87, 543–554 (2008).
Cougoule, C. et al. Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis. Blood 115, 1444–1452 (2010).
Ory, S., Brazier, H., Pawlak, G. & Blangy, A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur. J. Cell Biol. 87, 469–477 (2008).
Berdeaux, R. L., Diaz, B., Kim, L. & Martin, G. S. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J. Cell Biol. 166, 317–323 (2004).
Destaing, O., Saltel, F., Geminard, J. C., Jurdic, P. & Bard, F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14, 407–416 (2003).
Luxenburg, C. et al. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS ONE 2, e179 (2007).
Alexander, N. R. et al. Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 18, 1295–1299 (2008).
Schoumacher, M., Goldman, R. D., Louvard, D. & Vignjevic, D. M. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189, 541–556 (2010). A fascinating microscopic analysis of the temporal elements controlling invadopodium formation and movement through the basement membrane.
Furmaniak-Kazmierczak, E., Crawley, S. W., Carter, R. L., Maurice, D. H. & Cote, G. P. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ. Res. 100, 1328–1336 (2007).
Van Goethem, E. et al. Macrophage podosomes go 3D. Eur. J. Cell Biol. 90, 224–236 (2011).
Van Goethem, E., Poincloux, R., Gauffre, F., Maridonneau-Parini, I. & Le Cabec, V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J. Immunol. 184, 1049–1061 (2010).
Carman, C. V. et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity 26, 784–797 (2007).
Li, A. et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr. Biol. 20, 339–345 (2010).
Tolde, O., Rosel, D., Vesely, P., Folk, P. & Brabek, J. The structure of invadopodia in a complex 3D environment. Eur. J. Cell Biol. 89, 674–680 (2010).
Hotary, K., Allen, E., Punturieri, A., Yana, I. & Weiss, S. J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 149, 1309–1323 (2000). The first demonstration that MT1MMP is required for 3D but not 2D growth of cancer cells.
Linder, S., Nelson, D., Weiss, M. & Aepfelbacher, M. Wiskott–Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl Acad. Sci. USA 96, 9648–9653 (1999). The first report implicating a podosome-associated protein in a human disease.
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Blouw, B., Seals, D. F., Pass, I., Diaz, B. & Courtneidge, S. A. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur. J. Cell Biol. 87, 555–567 (2008).
Clark, E. S. et al. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene 28, 431–444 (2009).
Weaver, A. M. Cortactin in tumor invasiveness. Cancer Lett. 265, 157–166 (2008).
Yamaguchi, H. et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP–Arp2/3 complex pathway and cofilin. J. Cell Biol. 168, 441–452 (2005).
Gatesman, A., Walker, V. G., Baisden, J. M., Weed, S. A. & Flynn, D. C. Protein kinase Cα activates c-Src and induces podosome formation via AFAP-110. Mol. Cell Biol. 24, 7578–7597 (2004).
Yamaguchi, H. & Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652 (2007).
Zambonin-Zallone, A. et al. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β 3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 182, 645–652 (1989).
Helfrich, M. H. et al. β1 integrins and osteoclast function: involvement in collagen recognition and bone resorption. Bone 19, 317–328 (1996).
Mueller, S. C. & Chen, W. T. Cellular invasion into matrix beads: localization of β1 integrins and fibronectin to the invadopodia. J. Cell Sci. 99, 213–225 (1991).
Nakahara, H. et al. Activation of β1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 273, 199–212 (1998).
Nakamura, I. et al. Role of αvβ3 integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 112, 3985–3993 (1999).
Thomas, S. M. & Brugge, J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 (1997).
Lock, P., Abram, C. L., Gibson, T. & Courtneidge, S. A. A new method for isolating tyrosine kinase substrates used to identify Fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 17, 4346–4357 (1998).
Destaing, O. et al. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 19, 394–404 (2008).
Bromann, P. A., Korkaya, H. & Courtneidge, S. A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23, 7957–7968 (2004).
Obergfell, A. et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 157, 265–275 (2002).
Dorfleutner, A. et al. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J. Cell Sci. 121, 2394–2405 (2008).
Brown, D. I. & Griendling, K. K. Nox proteins in signal transduction. Free Radic. Biol. Med. 47, 1239–1253 (2009).
Grek, C. L. & Tew, K. D. Redox metabolism and malignancy. Curr. Opin. Pharmacol. 10, 362–368 (2010).
Gianni, D. et al. Novel p47phox-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2, ra54 (2009).
Giannoni, E., Buricchi, F., Raugei, G., Ramponi, G. & Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 25, 6391–6403 (2005).
Wu, W. S. et al. Reactive oxygen species mediated sustained activation of protein kinase Cα and extracellular signal-regulated kinase for migration of human hepatoma cell Hepg2. Mol. Cancer Res. 4, 747–758 (2006).
Meng, T. C., Fukada, T. & Tonks, N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 (2002).
Baranwal, S. & Alahari, S. K. miRNA control of tumor cell invasion and metastasis. Int. J. Cancer 126, 1283–1290 (2010).
Inui, M., Martello, G. & Piccolo, S. MicroRNA control of signal transduction. Nature Rev. Mol. Cell Biol. 11, 252–263 (2010).
Cordes, K. R. et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 (2009).
Elia, L. et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 16, 1590–1598 (2009).
Xin, M. et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 23, 2166–2178 (2009).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Chan, K. T., Cortesio, C. L. & Huttenlocher, A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 185, 357–370 (2009).
Liu, S., Yamashita, H., Weidow, B., Weaver, A. M. & Quaranta, V. Laminin-332–β1 integrin interactions negatively regulate invadopodia. J. Cell Physiol. 223, 134–142 (2010).
Vitale, S., Avizienyte, E., Brunton, V. G. & Frame, M. C. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly. Eur. J. Cell Biol. 87, 569–579 (2008).
Hauck, C. R., Hsia, D. A., Ilic, D. & Schlaepfer, D. D. v-Src SH3-enhanced interaction with focal adhesion kinase at β1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 277, 12487–12490 (2002).
Bruzzaniti, A. et al. Dynamin reduces Pyk2 Y402 phosphorylation and Src binding in osteoclasts. Mol. Cell Biol. 29, 3644–3656 (2009).
Duong, L. T. et al. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of αvβ3 integrin, and phosphorylated by Src kinase. J. Clin. Invest. 102, 881–892 (1998).
Gil-Henn, H. et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 178, 1053–1064 (2007).
Lakkakorpi, P. T., Bett, A. J., Lipfert, L., Rodan, G. A. & Duong le, T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 278, 11502–11512 (2003).
Oser, M. et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571–587 (2009). This paper provides a mechanism by which cortactin phosphorylation promotes invadopodium assembly.
Buschman, M. D. et al. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol. Biol. Cell 20, 1302–1311 (2009).
Poincloux, R., Lizarraga, F. & Chavrier, P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015–3024 (2009). A comprehensive review of the regulation of MT1MMP trafficking.
Wu, X., Gan, B., Yoo, Y. & Guan, J. L. FAK-mediated Src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev. Cell 9, 185–196 (2005).
Galvez, B. G., Matias-Roman, S., Yanez-Mo, M., Sanchez-Madrid, F. & Arroyo, A. G. ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 159, 509–521 (2002).
Bravo-Cordero, J. J. et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 26, 1499–1510 (2007).
Noritake, J., Watanabe, T., Sato, K., Wang, S. & Kaibuchi, K. IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 118, 2085–2092 (2005).
Sakurai-Yageta, M. et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 181, 985–998 (2008).
Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K. & Salmon, E. D. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nature Cell Biol. 1, 45–50 (1999).
Linder, S., Hufner, K., Wintergerst, U. & Aepfelbacher, M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 113, 4165–4176 (2000).
Wiesner, C., Faix, J., Himmel, M., Bentzien, F. & Linder, S. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood 116, 1559–1569 (2010).
Goode, B. L., Drubin, D. G. & Barnes, G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 12, 63–71 (2000).
Badowski, C. et al. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol. Biol. Cell 19, 633–645 (2008).
Granot-Attas, S., Luxenburg, C., Finkelshtein, E. & Elson, A. Protein tyrosine phosphatase-ɛ regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol. Biol. Cell 20, 4324–4334 (2009).
Cortesio, C. L. et al. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J. Cell Biol. 180, 957–971 (2008).
Chuang, Y. Y. et al. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 64, 8271–8275 (2004).
Mukhopadhyay, U. K. et al. Doubles game: Src–Stat3 versus p53–PTEN in cellular migration and invasion. Mol. Cell Biol. 30, 4980–4995 (2010).
Calle, Y., Carragher, N. O., Thrasher, A. J. & Jones, G. E. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J. Cell Sci. 119, 2375–2385 (2006).
Blanco, M. J. et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21, 3241–3246 (2002).
Come, C. et al. Snail and slugs play distinct roles during breast carcinoma progression. Clin. Cancer Res. 12, 5395–5402 (2006).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Kalluri, R. & Weinberg, R. A. The basics of epithelial–mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Eckert, M. A. et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372–386 (2011).
Iqbal, Z. et al. Disruption of the podosome adaptor protein TKS4 (SH3PXD2B) causes the skeletal dysplasia, eye, and cardiac abnormalities of Frank–ter Haar Syndrome. Am. J. Hum. Genet. 86, 254–261 (2010).
Baas, D., Malbouyres, M., Haftek-Terreau, Z., Le Guellec, D. & Ruggiero, F. Craniofacial cartilage morphogenesis requires zebrafish col11a1 activity. Matrix Biol. 28, 490–502 (2009).
Coyle, R. C., Latimer, A. & Jessen, J. R. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp. Cell Res. 314, 2150–2162 (2008).
Jopling, C. & den Hertog, J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 6, 426–431 (2005).
McCusker, C., Cousin, H., Neuner, R. & Alfandari, D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol. Biol. Cell 20, 78–89 (2009).
Neuner, R., Cousin, H., McCusker, C., Coyne, M. & Alfandari, D. Xenopus ADAM19 is involved in neural, neural crest and muscle development. Mech. Dev. 126, 240–255 (2009).
Sharma, D., Holets, L., Zhang, X. & Kinsey, W. H. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev. Biol. 285, 462–476 (2005).
Takatsuka, A. et al. Ablation of Csk in neural crest lineages causes corneal anomaly by deregulating collagen fibril organization and cell motility. Dev. Biol. 315, 474–488 (2008).
Cejudo-Martin, P. & Courtneidge, S. A. Podosomal proteins as causes of human syndromes: a role in craniofacial development? Genesis 49, 209–221 (2011).
Kirchhausen, T. Wiskott–Aldrich syndrome: a gene, a multifunctional protein and the beginnings of an explanation. Mol. Med. Today 4, 300–304 (1998).
Monypenny, J. et al. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur. J. Cell Biol. 90, 198–204 (2010).
Bonauer, A., Boon, R. A. & Dimmeler, S. Vascular microRNAs. Curr. Drug Targets 11, 943–949 (2010).
Busco, G. et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 24, 3903–3915 (2010).
Onodera, Y. et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 24, 963–973 (2005).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Hotary, K. B. et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33–45 (2003).
Scott, R. W. et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J. Cell Biol. 191, 169–185 (2010).
Mattila, P. K. & Lappalainen, P. Filopodia: molecular architecture and cellular functions. Nature Rev. Mol. Cell Biol. 9, 446–454 (2008).
Chhabra, E. S. & Higgs, H. N. The many faces of actin: matching assembly factors with cellular structures. Nature Cell Biol. 9, 1110–1121 (2007).
Raucher, D. et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton–plasma membrane adhesion. Cell 100, 221–228 (2000).
Nachmias, V. T., Kavaler, J. & Jacubowitz, S. Reversible association of myosin with the platelet cytoskeleton. Nature 313, 70–72 (1985).
Dikovsky, D., Bianco-Peled, H. & Seliktar, D. Defining the role of matrix compliance and proteolysis in three-dimensional cell spreading and remodeling. Biophys. J. 94, 2914–2925 (2008).
Raucher, D. & Sheetz, M. P. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J. Cell Biol. 148, 127–136 (2000).
Schober, J. M., Komarova, Y. A., Chaga, O. Y., Akhmanova, A. & Borisy, G. G. Microtubule- targeting-dependent reorganization of filopodia. J. Cell Sci. 120, 1235–1244 (2007).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Wolfenson, H., Henis, Y. I., Geiger, B. & Bershadsky, A. D. The heel and toe of the cell's foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton 66, 1017–1029 (2009).
Franz, C. M. & Muller, D. J. Analyzing focal adhesion structure by atomic force microscopy. J. Cell Sci. 118, 5315–5323 (2005).
Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A. & Geiger, B. Involvement of microtubules in the control of adhesion-depend ent signal transduction. Curr. Biol. 6, 1279–1289 (1996).
Chellaiah, M. A., Biswas, R. S., Yuen, D., Alvarez, U. M. & Hruska, K. A. Phosphatidylinositol 3, 4, 5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J. Biol. Chem. 276, 47434–47444 (2001).
Furlan, F. et al. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J. Bone Miner. Res. 22, 1387–1396 (2007).
Miyauchi, A. et al. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell Biol. 111, 2543–2552 (1990).
Monsky, W. L. et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 54, 5702–5710 (1994).
Nakahara, H. et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl Acad. Sci. USA 94, 7959–7964 (1997).
Sato, T. et al. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell Sci. 110, 589–596 (1997).
Acknowledgements
We are grateful to B. Diaz, M. Buschman and C. Gould for reading the manuscript and to J. Tsai for help in figure preparation. The Courtneidge laboratory is supported by grants from the National Cancer Institute, National Institutes of Health, USA, and the Mathers Foundation, New York, USA. D.A.M. is supported by training grant T32CA121949 and The American Cancer Society North Texas Postdoctoral Fellowship, 2008 (North Texas Creating Tomorrow's Miracles).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- SH3 domains
-
Small, independently folding protein domains that mediate binding to Pro-rich motifs in other proteins.
- Total internal reflection fluorescence
-
TIRF microscopy is a technique that is often used to visualize molecules and structures at or near to the ventral surface of a cell.
- PX domain
-
A protein domain (∼120 amino acids) that associates with phosphatidylinositol lipids.
- Podosome belt
-
A region of podosome fusion at the periphery of osteoclasts that are cultured in two dimensions.
- Outside–in signalling
-
The transmission of extracellular signals to the cytoplasm by the engagement of integrin receptors with the extracellular matrix.
- Inside–out signalling
-
The transmission of the status of the cell to the extracellular space through integrin receptors.
- Actin cloud
-
A region of high concentration of actin that surrounds podosome cores in osteoclasts.
- Barbed end
-
The ends of filamentous actin polymers to which globular actin monomers are attached.
- Epithelial–mesenchymal transition
-
The transformation of an epithelial cell into a mesenchymal cell, which has migratory and invasive properties.
Rights and permissions
About this article
Cite this article
Murphy, D., Courtneidge, S. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 12, 413–426 (2011). https://doi.org/10.1038/nrm3141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3141
This article is cited by
-
Microglia degrade Tau oligomers deposit via purinergic P2Y12-associated podosome and filopodia formation and induce chemotaxis
Cell & Bioscience (2023)
-
RPL21 interacts with LAMP3 to promote colorectal cancer invasion and metastasis by regulating focal adhesion formation
Cellular & Molecular Biology Letters (2023)
-
MMP14 expression levels accurately predict the presence of extranodal extensions in oral squamous cell carcinoma: a retrospective cohort study
BMC Cancer (2023)
-
Chemo-mechanical diffusion waves explain collective dynamics of immune cell podosomes
Nature Communications (2023)
-
Deciphering the involvement of the Hippo pathway co-regulators, YAP/TAZ in invadopodia formation and matrix degradation
Cell Death & Disease (2023)