Key Points
-
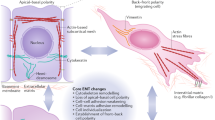
Epithelial and mesenchymal cells have distinct characteristics. These cell types can be partially or fully interconverted through the processes of epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition (MET).
-
EMT is controlled by various extracellular triggers. The intracellular pathways that are activated by these triggers exhibit extensive crosstalk and have many common endpoints.
-
Biophysics as well as cell- and molecular-biology approaches have been combined to provide many novel insights into the process of EMT.
-
EMT has an important role in many embryological processes. Examples include gastrulation, neural-crest development and heart-valve formation.
-
There is mounting evidence that EMT processes are involved in several pathological processes, including would healing, fibrosis and cancer.
-
Better model systems and the identification of genes that mark specific EMT events are required for further progress in this field.
Abstract
Epithelial–mesenchymal transition is an indispensable mechanism during morphogenesis, as without mesenchymal cells, tissues and organs will never be formed. However, epithelial-cell plasticity, coupled to the transient or permanent formation of mesenchyme, goes far beyond the problem of cell-lineage segregation. Understanding how mesenchymal cells arise from an epithelial default status will also have a strong impact in unravelling the mechanisms that control fibrosis and cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schock, F. & Perrimon, N. Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 18, 463–493 (2002).
Thompson, E. W., Newgreen, D. F. & Tarin, D. Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Res. 65, 5991–5995 (2005).
Friedl, P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14–23 (2004).
Christ, B. & Ordahl, C. P. Early stages of chick somite development. Anat. Embryol. (Berl) 191, 381–396 (1995).
Funayama, N., Sato, Y., Matsumoto, K., Ogura, T. & Takahashi, Y. Coelom formation: binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development 126, 4129–4138 (1999).
Locascio, A. & Nieto, M. A. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev. 11, 464–469 (2001).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nature Rev. Mol. Cell Biol. 3, 155–166 (2002).
Gilbert S. F. Developmental Biology, 7th edn (Sinauer Associates Inc., 2003).
Gershengorn, M. C., et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science 306, 2261–2264 (2004).
Radisky, D. C. Epithelial–mesenchymal transition. J. Cell Sci. 118, 4325–4326 (2005).
Peinado, H., Portillo, F. & Cano, A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 48, 365–375 (2004).
Wang, J. et al. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev. Biol. 286, 299–310 (2005).
Zavadil, J. & Bottinger, E. P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Savagner, P. Leaving the neighborhood: molecular mechanisms involved during epithelial–mesenchymal transition. Bioessays 23, 912–923 (2001).
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002). A comprehensive review of the basics of EMT.
Thiery, J. P. Epithelial–mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 (2003).
Zoltan-Jones, A., Huang, L., Ghatak, S. & Toole, B. P. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J. Biol. Chem. 278, 45801–45810 (2003).
Barrallo-Gimeno, A. & Nieto, M. A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132, 3151–3161 (2005). An excellent review of the different facets of the snail genes.
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Grille, S. J. et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63, 2172–2178 (2003).
Beals, C. R., Sheridan, C. M., Turck, C. W., Gardner, P. & Crabtree, G. R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 (1997).
Zhou, B. P. et al. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nature Cell Biol. 6, 931–940 (2004).
Yang, Z. et al. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 65, 3179–3184 (2005).
Bhowmick, N. A., Zent, R., Ghiassi, M., McDonnell, M. & Moses, H. L. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 276, 46707–46713 (2001).
Prunier, C. & Howe, P. H. Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT). J. Biol. Chem. 280, 17540–17548 (2005).
Valles, A. M., Boyer, B., Tarone, G. & Thiery, J. P. α2 β1 integrin is required for the collagen and FGF-1 induced cell dispersion in a rat bladder carcinoma cell line. Cell Adhes. Commun. 4, 187–199 (1996).
Balzac, F. et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J. Cell Sci. 118, 4765–4783 (2005).
Oloumi, A., McPhee, T. & Dedhar, S. Regulation of E-cadherin expression and β-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochem. Biophys. Acta 1691, 1–15 (2004).
Li, Y., Yang, J., Dai, C., Wu, C. & Liu, Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112, 503–516 (2003).
Gimond, C. et al. Induction of cell scattering by expression of β1 integrins in β1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J. Cell Biol. 147, 1325–1340 (1999).
Avizienyte, E. & Frame, M. C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 17, 542–547 (2005).
Yano, H. et al. Roles played by a subset of integrin signaling molecules in cadherin-based cell–cell adhesion. J. Cell Biol. 166, 283–295 (2004).
Chu, Y. S. et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 167, 1183–1194 (2004).
Perez-Moreno, M., Jamora, C. & Fuchs, E. Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535–548 (2003).
Ebnet, K., Suzuki, A., Ohno, S. & Vestweber, D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 117, 19–29 (2004).
Takai, Y. & Nakanishi, H. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116, 17–27 (2003).
Martinez-Rico, C. et al. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and-3. J. Biol. Chem. 280, 4753–4760 (2005).
Chu, Y. S. et al. Prototypical type-I E-cadherin and type-II cadherin-7 mediate very distinct adhesiveness through their extracellular domain. J. Biol. Chem. Oct 16 2005 (10.1074/jbc.M506185200).
Barrios-Rodiles, M. et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307, 1621–1625 (2005).
Ozdamar, B. et al. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307, 1603–1609 (2005).
Wang, H. R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003). Exploited an advanced technique to discover new critical partners in tight junctions that couple signalling and morphogenesis.
Fernandez-Serra, M., Consales, C., Livigni, A. & Arnone, M. I. Role of the ERK-mediated signaling pathway in mesenchyme formation and differentiation in the sea urchin embryo. Dev. Biol. 268, 384–402 (2004).
Rottinger, E., Besnardeau, L. & Lepage, T. A Raf/MEK/ERK signaling pathway is required for development of the sea urchin embryo micromere lineage through phosphorylation of the transcription factor Ets. Development 131, 1075–1087 (2004).
Oda, H., Tsukita, S. & Takeichi, M. Dynamic behavior of the cadherin-based cell–cell adhesion system during Drosophila gastrulation. Dev. Biol. 203, 435–450 (1998).
Smallhorn, M., Murray, M. J. & Saint, R. The epithelial–mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development 131, 2641–2651 (2004).
Yamashita, S. et al. Zinc transporter LIVI controls epithelial–mesenchymal transition in zebrafish gastrula organizer. Nature 429, 298–302 (2004).
Ciruna, B. & Rossant, J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37–49 (2001).
Carver, E. A., Jiang, R., Lan, Y., Oram, K. F. & Gridley, T. The mouse snail gene encodes a key regulator of the epithelial–mesenchymal transition. Mol. Cell Biol. 21, 8184–8188 (2001).
Kemler, R. et al. Stabilization of β-catenin in the mouse zygote leads to premature epithelial–mesenchymal transition in the epiblast. Development 131, 5817–5824 (2004).
Huelsken, J. et al. Requirement for β-catenin in anterior–posterior axis formation in mice. J. Cell Biol. 148, 567–578 (2000).
Lickert, H. et al. Formation of multiple hearts in mice following deletion of β-catenin in the embryonic endoderm. Dev. Cell 3, 171–181 (2002).
Meulemans, D. & Bronner-Fraser, M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291–299 (2004). A comprehensive review that provides a basis for stimulating new evolution and development studies.
del Barrio, M. G. & Nieto, M. A. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129, 1583–1593 (2002).
Vallin, J. et al. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/β-catenin signaling. J. Biol. Chem. 276, 30350–30358 (2001).
Cheung, M. et al. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179–192 (2005). An advanced study that addresses the complexity of signalling pathways in neural-crest ontogeny in vivo.
Morales, A. V., Barbas, J. A. & Nieto, M. A. How to become neural crest: from segregation to delamination. Semin. Cell Dev. Biol. 16, 655–662 (2005).
Peinado, H., Quintanilla, M. & Cano, A. Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 278, 21113–21123 (2003).
Timmerman, L. A. et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 18, 99–115 (2004).
Nakajima, Y., Yamagishi, T., Hokari, S. & Nakamura, H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP). Anat. Rec. 258, 119–127 (2000).
Zavadil, J., Cermak, L., Soto-Nieves, N. & Bottinger, E. P. Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 23, 1155–1165 (2004). A well-designed study to investigate a complex network of interactions in EMT.
Woodley, D. T. Reepithelialization. in The molecular and cellular biology of wound healing (ed Clarke, A. F.) 339–354 (Plenum Press, New York, 1998).
Martin, P. Wound healing — aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Savagner, P. et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J. Cell Physiol. 202, 858–866 (2005).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Kalluri, R. & Neilson, E. G. Epithelial–mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112, 1776–1784 (2003).
Strutz, F. et al. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 130, 393–405 (1995).
Ng, Y. Y. et al. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 54, 864–876 (1998).
Yang, J. & Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 159, 1465–1475 (2001).
Jinde, K. et al. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am. J. Kidney Dis. 38, 761–769 (2001).
Rastaldi, M. P. et al. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 62, 137–146 (2002).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Yang, J. et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Invest. 110, 1525–1538 (2002).
Yanez-Mo, M. et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348, 403–413 (2003).
Aguilera, A., Yanez-Mo, M., Selgas, R., Sanchez-Madrid, F. & Lopez-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 6, 262–268 (2005).
Margetts, P. J. et al. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 16, 425–436 (2005).
Salez, F. et al. Transforming growth factor-β1 in sarcoidosis. Eur. Respir. J. 12, 913–919 (1998).
Khalil, N. et al. Regulation of the effects of TGF-β1 by activation of latent TGF-β1 and differential expression of TGF-β receptors (TβR-I and TβR-II) in idiopathic pulmonary fibrosis. Thorax 56, 907–915 (2001).
Yao, H. W., Xie, Q. M., Chen, J. Q., Deng, Y. M. & Tang, H. F. TGF-β1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 76, 29–37 (2004).
Willis, B. C. et al. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor-β1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 166, 1321–1332 (2005).
de Iongh, R. U., Wederell, E., Lovicu, F. J. & McAvoy, J. W. Transforming growth factor-β-induced epithelial–mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs 179, 43–55 (2005).
Flanders, K. C. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 85, 47–64 (2004).
Saika, S. et al. Smad3 signaling is required for epithelial–mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 164, 651–663 (2004).
Saika, S. et al. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab. Invest. 84, 1245–1258 (2004).
Sleeman, J. P. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 157, 55–81 (2000).
Tarin, D., Thompson, E. W. & Newgreen, D. F. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 65, 5996–6000 (2005).
De Craene, B. et al. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 65, 6237–6244 (2005).
Affolter, M. et al. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11–18 (2003).
Nelson, C. M. & Bissell, M. J. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 15, 342–352 (2005).
O'Brien, P. M. et al. Immunoglobulin genes expressed by B-lymphocytes infiltrating cervical carcinomas show evidence of antigen-driven selection. Cancer Immunol. Immunother. 50, 523–532 (2001).
Debnath, J. & Brugge, J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nature Rev. Cancer 5, 675–688 (2005).
Savagner, P., Valles, A. M., Jouanneau, J., Yamada, K. M. & Thiery, J. P. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial–mesenchymal transition in rat bladder carcinoma cells. Mol. Biol. Cell 5, 851–862 (1994).
Pietri, T. et al. Conditional β1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development 131, 3871–3883 (2004).
Moody, S. E. et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 (2005). A remarkable model for the role of EMT in breast cancer progression.
Petersen, O. W. et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am. J. Pathol. 162, 391–402 (2003).
Xue, C., Plieth, D., Venkov, C., Xu, C. & Neilson, E. G. The gatekeeper effect of epithelial–mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 63, 3386–3394 (2003).
Perl, A. K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (1998).
Dhawan, P. et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Invest. 115, 1765–1776 (2005).
Huber, M. A. et al. NF-κB is essential for epithelial–mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Acknowledgements
This work was supported, in part, by the European Union under the auspices of the European Economic Comunity Framework Programme 6 Specific Targeted Research Project BRECOSM (Breast Cancer Metastasis). This review is dedicated to the memory of Professor Shoichiro Tsukita of Kyoto University who passed away in December 2005.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mesoderm
-
In animals with three tissue layers, the mesoderm is the middle layer of tissue, lying between the ectoderm and the endoderm. In vertebrates, it forms the skeleton, muscles, heart, spleen, kidney and other internal organs.
- Endoderm
-
The innermost germ layer of the developing embryo. It gives rise to the lungs, digestive tract, thyroid, thymus, liver and pancreas.
- Phyla
-
Large groups of species that share the same body plan. The animal kingdom is composed of about 30 phyla including Porifera, Cnidaria, Arthropoda, Echinodermata, and Chordata, which includes the Vertebrata as a subphylum.
- Porifera
-
The most primitive phylum of the animal kingdom, it includes sponges.
- Cnidaria
-
Radially symmetrical animals that form a phylum that includes jellyfish, corals, hydra and anemonies.
- Blastula stage
-
An early-stage embryo that is composed of a hollow ball of cells.
- Primitive colonial protozoans
-
Single-celled organisms that live in colonies — they might be the organisms from which Porifera developed.
- Diploblastic
-
Animals that are composed of two cell layers. They belong to the phylum Cnidaria.
- Ectoderm
-
The outermost of the three primary germ layers of the embryo, from which the skin, nerve tissue and sensory organs develop.
- Mesenchyme
-
Embryonic tissue that is composed of loosely organized, unpolarized cells of both mesodermal and ectodermal (for example, neural crest) origin, with a component-rich extracellular matrix.
- Organizer
-
A small dorsal region of the vertebrate gastrula-stage embryo that has the remarkable capacity to organize a complete embryonic body plan. Hilde Mangold and Hans Spemann first identified the organizer in amphibian embryos using tissue transplantation.
- Mesendoderm
-
Cells that form early during gastrulation in the vertebrate and are destined to give rise to mesodermal and endodermal derivatives.
- Rostrocaudal
-
The anterior–posterior (head to tail) polarity of animals.
- Neural crest
-
A transient embryonic structure of vertebrates that appears in the ectoderm at the junction between the neural plate and lateral ectoderm. This structure gives rise to many distinct derivatives following precise migratory routes at each axial level. The derivatives include cranio–facial structures (cartilage, bone, muscles), melanocytes, adrenal medulla, and cells of the sensory and autonomic nervous systems.
- Basement membrane
-
An extracellular-matrix structure that can be visualized by light microscopy and lines the basal side of epithelia.
Rights and permissions
About this article
Cite this article
Thiery, J., Sleeman, J. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7, 131–142 (2006). https://doi.org/10.1038/nrm1835
Issue Date:
DOI: https://doi.org/10.1038/nrm1835
This article is cited by
-
Co-expression of Twist and Snai1: predictor of poor prognosis and biomarker of treatment resistance in untreated prostate cancer
Molecular Biology Reports (2024)
-
The mechanism of the contribution of ICAM-1 to epithelial–mesenchymal transition (EMT) in bladder cancer
Human Cell (2024)
-
IGFBP2 drives epithelial-mesenchymal transition in hepatocellular carcinoma via activating the Wnt/β-catenin pathway
Infectious Agents and Cancer (2023)
-
The promoting effect and mechanism of Nrf2 on cell metastasis in cervical cancer
Journal of Translational Medicine (2023)
-
Epithelial cell adhesion molecule (EpCAM) regulates HGFR signaling to promote colon cancer progression and metastasis
Journal of Translational Medicine (2023)