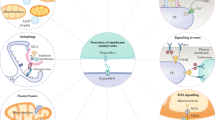
Key Points
-
Annexins are a multigene family of Ca2+-regulated proteins that are characterized by a unique Ca2+- and membrane-binding module — the annexin core domain. This core domain enables Ca2+-bound annexins to peripherally dock onto membranes that contain negatively charged phospholipids.
-
Each annexin contains a second, highly varible region — the N-terminal interaction domain. It harbours binding sites for cytoplasmic protein ligands that can be targeted to membranes through the annexin-core-mediated phospholipid interaction.
-
Membrane-bound annexins can form lateral self-assemblies that affect the mobility and organization of membrane lipids. Such activities probably regulate membrane-related processes like membrane-domain organization and membrane transport in endocytosis and exocytosis.
-
Interfering with intracellular annexin function, by overexpressing mutants or by using RNA-interference-mediated downregulation, has different effects depending on the annexin being targeted. These include effects on actin assemblies at cellular membranes, the organization of endosomal subcompartments, Ca2+-regulated exocytosis and midbody formation during cytokinesis.
-
Some annexins can also occur extracellularly and can have functions outside cells. Their release is not fully understood, but probably follows non-classical secretion pathways.
-
Extracellular annexin functions that have been substantiated by mouse knockout models are anti-inflammatory and fibrinolytic activities. These are probably mediated through specific cell-surface interactions with chemoattractant receptors on cells of the immune system and key enzymes of the fibrinolytic cascade, respectively.
Abstract
Eukaryotic cells contain various Ca2+-effector proteins that mediate cellular responses to changes in intracellular Ca2+ levels. A unique class of these proteins — annexins — can bind to certain membrane phospholipids in a Ca2+-dependent manner, providing a link between Ca2+ signalling and membrane functions. By forming networks on the membrane surface, annexins can function as organizers of membrane domains and membrane-recruitment platforms for proteins with which they interact. These and related properties enable annexins to participate in several otherwise unrelated events that range from membrane dynamics to cell differentiation and migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Huber, R., Römisch, J. & Paques, E. P. The crystal and molecular structure of human annexin V, an anticoagulant calcium, membrane binding protein. EMBO J. 9, 3867–3874 (1990). The first crystal structure of an annexin (annexin A5) shows the characteristic fold of the annexin core.
Liemann, S. & Lewit-Bentley, A. Annexins: a novel family of calcium- and membrane-binding proteins in search of a function. Structure 3, 233–237 (1995).
Swairjo, M. A. & Seaton, B. A. Annexin structure and membrane interactions: a molecular perspective. Annu. Rev. Biophys. Biomol. Struct. 23, 193–213 (1994).
Swairjo, M. A., Concha, N. O., Kaetzel, M. A., Dedman, J. R. & Seaton, B. A. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nature Struct. Biol. 2, 968–974 (1995).
Huber, R., Schneider, M., Mayr, I., Römisch, J. & Paques, E. P. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 Å resolution. FEBS Lett. 275, 15–21 (1990).
Weng, X. et al. Crystal structure of human annexin I at 2.5 Å resolution. Protein Sci. 2, 448–458 (1993).
Concha, N. O., Head, J. F., Kaetzel, M. A., Dedman, J. R. & Seaton, B. A. Rat annexin V crystal structure: Ca2+-induced conformational changes. Science 261, 1321–1324 (1993).
Lewit-Bentley, A., Morera, S., Huber, R. & Bodo, G. The effect of metal binding on the structure of annexin V and implications for membrane binding. Eur. J. Biochem. 210, 73–77 (1992).
Wice, B. M. & Gordon, J. I. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: characterization of a novel gut-specific N-myristoylated annexin. J. Cell Biol. 116, 405–422 (1992).
Langen, R., Isas, J. M., Hubbel, W. L. & Haigler, H. T. A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl Acad. Sci. USA 95, 14060–14065 (1998). This study used a site-specific spin-labelling approach to describe a transmembrane form of the Hydra annexin B12 that is proposed to result from a conformational rearrangement induced by acidic pH values.
Kubista, H., Hawkins, T. E., Patel, D. R., Haigler, H. T. & Moss, S. E. Annexin 5 mediates peroxide-induced Ca2+ influx in B cells. Curr. Biol. 9, 1403–1406 (1999).
Huber, R., Berendes, R., Burger, A., Luecke, H. & Karshikov, A. in The Annexins (ed. Moss, S. E.) 105–124 (Portland, London, 1992).
Rosengarth, A. & Luecke, H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J. Mol. Biol. 326, 1317–1325 (2003).
Gerke, V. & Moss, S. E. Annexins: from structure to function. Phys. Rev. 82, 331–371 (2002).
Rety, S. et al. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nature Struct. Biol. 6, 89–95 (1999).
Rety, S. et al. Structural basis of the Ca2+-dependent association between S100C (S100A11) and its target, the N-terminal part of annexin I. Structure 8, 175–184 (2000).
Lewit-Bentley, A., Rety, S., Sopkova-de Oliveira Santos, J. & Gerke, V. S100–annexin complexes: some insights from structural studies. Cell Biol. Int. 24, 799–802 (2000).
Moss, S. E. & Morgan, R. O. The annexins. Genome Biol. 5, 219 (2004).
Avila-Sakar, A. J., Creutz, C. E. & Kretsinger, R. H. Crystal structure of bovine annexin VI in a calcium-bound state. Biochim. Biophys. Acta 1387, 103–116 (1998).
Avila-Sakar, A. J., Kretsinger, R. H. & Creutz, C. E. Membrane-bound 3D structures reveal the intrinsic flexibility of annexin VI. J. Struct. Biol. 130, 54–62 (2000).
Freye-Minks, C., Kretsinger, R. H. & Creutz, C. E. Structural and dynamic changes in human annexin VI induced by a phosphorylation-mimicking mutation, T356D. Biochemistry 42, 620–630 (2003).
Kaetzel, M. A. et al. Phosphorylation mutants elucidate the mechanism of annexin IV-mediated membrane aggregation. Biochemistry 40, 4192–4199 (2001).
Oling, F. et al. Structure of membrane-bound annexin A5 trimers: a hybrid cryo-EM–X-ray crystallography study. J. Mol. Biol. 304, 561–573 (2000).
Pigault, C., Follenius, W. A., Schmutz, M., Freyssinet, J. M. & Brisson, A. Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. J. Mol. Biol. 236, 199–208 (1994).
Voges, D. et al. Three-dimensional structure of membrane-bound annexin V. A correlative electron microscopy–X-ray crystallography study. J. Mol. Biol. 238, 199–213 (1994).
Reviakine, I., Bergsma-Schutter, W. & Brisson, A. Growth of protein 2-D crystals on supported planar lipid bilayers imaged in situ by AFM. J. Struct. Biol. 121, 356–361 (1998). Using electron microscopy and atomic-force microscopy, references 24–26 reveal the formation of ordered annexin A5 arrays on planar lipid bilayers that contain acidic phospholipids.
Kenis, H. et al. Cell surface expressed phosphatidylserine and annexin A5 open a novel portal of cell entry. J. Biol. Chem. 279, 52623–52629 (2004).
Janshoff, A., Ross, M., Gerke, V. & Steinem, C. Visualization of annexin I binding to calcium-induced phosphatidylserine domains. Chembiochem. 2, 587–590 (2001).
Menke, M., Ross, M., Gerke, V. & Steinem, C. The molecular arrangement of membrane-bound annexin A2–S100A10 tetramer as revealed by scanning force microscopy. Chembiochem 5, 1003–1006 (2004).
Junker, M. & Creutz, C. E. Endonexin (annexin IV)-mediated lateral segregation of phosphatidylglycerol in phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry 32, 9968–9974 (1993).
Wang, W. & Creutz, C. E. Role of the amino-terminal domain in regulating interactions of annexin I with membranes: effects of amino-terminal truncation and mutagenesis of the phosphorylation sites. Biochemistry 33, 275–282 (1994).
Drust, D. S. & Creutz, C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature 331, 88–91 (1988).
Lambert, O., Gerke, V., Bader, M. F., Porte, F. & Brisson, A. Structural analysis of junctions formed between lipid membranes and several annexins by cryo electron microscopy. J. Mol. Biol. 272, 42–55 (1997).
Creutz, C. E., Snyder, S. L., Daigle, S. N. & Redick, J. Identification, localization, and functional implications of an abundant nematode annexin. J. Cell Biol. 132, 1079–1092 (1996). This study describes the identification of the main C. elegans annexin, NEX-1, and its striking enrichment on the cytoplasmic surface of membranes of the spermathecal valve.
Daigle, S. N. & Creutz, C. E. Transcription, biochemistry and localization of nematode annexins. J. Cell Sci. 112, 1901–1913 (1999).
Wang, W. & Creutz, C. E. Regulation of the chromaffin granule aggregating activity of annexin I by phosphorylation. Biochemistry 31, 9934–9939 (1992).
Johnstone, S. A., Hubaishy, I. & Waisman, D. M. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J. Biol. Chem. 267, 25976–25981 (1992).
Caohuy, H. & Pollard, H. B. Activation of annexin 7 by protein kinase C in vitro and in vivo. J. Biol. Chem. 276, 12813–12821 (2001).
Merrifield, C. J. et al. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol. 1, 72–74 (1999).
Merrifield, C. J. et al. Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr. Biol. 11, 1136–1141 (2001).
Zobiack, N. et al. Cell surface attachment of pedestal forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin 2. J. Cell Sci. 115, 91–98 (2002).
Hayes, M. J. et al. Annexin 2 binding to phosphatidylinositol 4,5 bisphosphate on endocytic vesicles is regulated by the stress response pathway. J. Biol. Chem. 279, 14157–14164 (2004).
Rescher, U., Ruhe, D., Ludwig, C., Zobiack, N. & Gerke, V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J. Cell Sci. 117, 3473–3480 (2004).
Rescher, U. & Gerke, V. Annexins — unique membrane binding proteins with diverse functions. J. Cell Sci. 117, 2631–2639 (2004).
Hayes, M. J., Rescher, U., Gerke, V. & Moss, S. E. Annexin–actin interactions. Traffic 5, 571–576 (2004).
Babiychuk, E. B. & Draeger, A. Annexins in cell membrane dynamics: Ca2+-regulated association of lipid microdomains. J. Cell Biol. 150, 1113–1123 (2000).
Tomas, A., Futter, C. & Moss, S. E. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol. 165, 813–822 (2004). By employing RNA interference to downregulate annexin A11, these authors show that this annexin functions in the terminal phase of cytokinesis, possibly participating in the delivery of new membrane material that is required for abscission.
Gerke, V. & Moss, S. E. Annexins and membrane dynamics. Biochim. Biophys. Acta 1357, 129–154 (1997).
Eberhard, D. A., Karns, L. R., VandenBerg, S. R. & Creutz, C. E. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J. Cell Sci. 114, 3155–3166 (2001).
Mohiti, J., Caswell, A. M. & Walker, J. H. The nuclear location of annexin V in the human osteosarcoma cell line MG-63 depends on serum factors and tyrosine kinase signaling pathways. Exp. Cell Res. 234, 98–104 (1997).
Sacre, S. M. & Moss, S. E. Intracellular localization of endothelial cell annexins is differentially regulated by oxidative stress. Exp. Cell Res. 274, 254–263 (2002).
Mizutani, A. et al. CAP-50, a newly identified annexin, localizes in nuclei of cultured fibroblast 3Y1 cells. J. Biol. Chem. 267, 13498–13504 (1992).
Tomas, A. & Moss, S. E. Calcium- and cell cycle-dependent association of annexin 11 with the nuclear envelope. J. Biol. Chem. 278, 20210–20216 (2003).
Vedeler, A. & Hollas, H. Annexin II is associated with mRNA which may constitute a distinct subpopulation. Biochem. J. 348, 565–572 (2000).
Filipenko, N. R., Macleod, T. J., Yoon, C. S. & Waisman, D. M. Annexin A2 is a novel RNA binding protein. J. Biol. Chem. 279, 8723–8731 (2004).
Chapman, L. P., Epton, M. J., Buckingham, J. C., Morris, J. F. & Christian, H. C. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology 144, 1062–1073 (2003).
Danielsen, E. M., Van Deurs, B. & Hansen, G. H. 'Nonclassical' secretion of annexin A2 to the lumenal side of the enterocyte brush border membrane. Biochemistry 42, 14670–14676 (2003).
Faure, A. V., Migne, C., Devilliers, G. & Ayala-Sanmartin, J. Annexin 2 'secretion' accompanying exocytosis of chromaffin cells: possible mechanisms of annexin release. Exp. Cell Res. 276, 79–89 (2002).
Genge, B. R., Wu, L. N. & Wuthier, R. E. Differential fractionation of matrix vesicle proteins. Further characterization of the acidic phospholipid-dependent Ca2+-binding proteins. J. Biol. Chem. 265, 4703–4710 (1990).
Deora, A. B., Kreitzer, G., Jacovina, A. T. & Hajjar, K. A. An annexin 2 phosphorylation switch mediates its p11-dependent translocation to the cell surface. J. Biol. Chem. 279, 43411–43418 (2004).
Creutz, C. E. The annexins and exocytosis. Science 258, 924–931 (1992).
Creutz, C. E., Pazoles, C. J. & Pollard, H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of chromaffin granules. J. Biol. Chem. 253, 2858–2866 (1978). The founding paper of the entire annexin field, which describes the first identification of an annexin (synexin; now known as annexin A7). It also reports a hallmark activity for this annexin — Ca2+-dependent vesicle aggregation.
Creutz, C. E. in Annexins: Biological Importance and Annexin-Related Pathologies (ed. Bandorowicz-Pikula, J.) 1–20 (Landes Bioscience, Georgetown, 2003).
Creutz, C. E. et al. Identification of chromaffin granule-binding proteins. J. Biol. Chem. 262, 1860–1868 (1987).
Creutz, C. E. Cis-unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J. Cell Biol. 91, 247–256 (1981).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Ali, S. M., Geisow, M. J. & Burgoyne, R. D. A role for calpactin in calcium dependent exocytosis in adrenal chromaffin cells. Nature 340, 313–315 (1989).
Sarafian, T., Pradel, L. A., Henry, J. P., Aunis, D. & Bader, M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J. Cell Biol. 114, 1135–1147 (1991).
Chasserot-Golaz, S. et al. Annexin II in exocytosis: catecholamin secretion requires the transolcation of p36 to the subplasmalemmal region in chromaffin cells. J. Cell Biol. 133, 1217–1236 (1996).
Graham, M. E., Gerke, V. & Burgoyne, R. D. Modification of annexin II expression in PC12 cell line does not affect Ca2+-dependent exocytosis. Mol. Biol. Cell 8, 431–442 (1997).
König, J., Prenen, J., Nilius, B. & Gerke, V. The annexin II–p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J. Biol. Chem. 273, 19679–19684 (1998).
Knop, M., Aareskjold, E., Bode, G. & Gerke, V. Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J. 23, 2982–2992 (2004). Using different approaches, references 67, 68 and 72 show that the annexin-A2–S100A10 complex is involved in certain exocytosis events, namely the Ca2+-evoked exocytosis of chromaffin granules in adrenal chromaffin cells and of Weibel–Palade bodies in endothelial cells.
Lafont, F., Lecat, S., Verkande, P. & Simons, K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol. 142, 1413–1427 (1998). An elegant paper showing that in polarized epithelial cells the myristoylated form of annexin A13b functions in the formation and apical delivery of transport vesicles that are rich in lipid microdomains.
Tucker, W. C., Weber, T. & Chapman, E. R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Damer, C. K. & Creutz, C. E. Synergistic membrane interactions of the two C2 domains of synaptotagmin. J. Biol. Chem. 269, 31115–31123 (1994).
Emans, N. et al. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 120, 1357–1369 (1993).
Harder, T. & Gerke, V. The subcellular distribution of early endosomes is affected by the annexin II2p112 complex. J. Cell Biol. 123, 1119–1132 (1993).
Jost, M., Zeuschner, D., Seemann, J., Weber, K. & Gerke, V. Identification and characterization of a novel type of annexin–membrane interaction: Ca2+ is not required for the association of annexin II with endosomal membranes. J. Cell Sci. 110, 221–228 (1997).
Seemann, J., Weber, K., Osborn, M., Parton, R. G. & Gerke, V. The association of annexin I with early endosomes is regulated by Ca2+ and requires an intact N-terminal domain. Mol. Biol. Cell 7, 1359–1374 (1996).
Mayran, N., Parton, R. G. & Gruenberg, J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 22, 3242–3253 (2003).
Gruenberg, J. & Stenmark, H. The biogenesis of multivesicular endosomes. Nature Rev. Mol. Cell Biol. 5, 317–323 (2004).
Zobiack, N., Rescher, U., Ludwig, C., Zeuschner, D. & Gerke, V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 14, 4896–4908 (2003). References 80 and 82 used the RNA-interference-mediated depletion of annexin A2 to show that this protein is involved in early endosome dynamics — that is, in maintaining the morphological appearance of recycling endosomes and in the biogenesis of multivesicular endosomes, respectively.
Harder, T., Kellner, R., Parton, R. G. & Gruenberg, J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol. Biol. Cell 8, 533–545 (1997).
Zeuschner, D., Stoorvogel, W. & Gerke, V. Association of annexin 2 with recycling endosomes requires either calcium or cholesterol. Eur. J. Cell Biol. 80, 499–507 (2001).
Futter, C. E., Felder, S., Schlessinger, J., Ullrich, A. & Hopkins, C. R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell Biol. 120, 77–83 (1993).
Bache, K. G., Brech, A., Mehlum, A. & Stenmark, H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 162, 435–442 (2003).
Kamal, A., Ying, Y. & Anderson, R. G. Annexin VI-mediated loss of spectrin during coated pit budding is coupled to delivery of LDL to lysosomes. J. Cell Biol. 142, 937–947 (1998).
Grewal, T. et al. Annexin VI stimulates endocytosis and is involved in the trafficking of LDL to the prelysosmal compartment. J. Biol. Chem. 275, 33806–33813 (2000).
Pons, M. et al. Evidence for the involvement of annexin 6 in the trafficking between the endocytic compartment and lysosomes. Exp. Cell Res. 269, 13–22 (2001).
Kaetzel, M. A. & Dedman, J. R. Annexin VI regulation of cardiac function. Biochem. Biophys. Res. Commun. 322, 1171–1177 (2004).
Smythe, E., Smith, P. D., Jacob, S. M., Theobald, J. & Moss, S. E. Endocytosis occurs independently of annexin VI in human A431 cells. J. Cell Biol. 124, 301–306 (1994).
Matveev, S., Uittenbogaard, A., van Der Westhuyzen, D. & Smart, E. J. Caveolin-1 negatively regulates SR-BI mediated selective uptake of high-density lipoprotein-derived cholesteryl ester. Eur. J. Biochem. 268, 5609–5916 (2001).
Uittenbogaard, A., Everson, W. V., Matveev, S. V. & Smart, E. J. Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin-annexin II lipid-protein complex. J. Biol. Chem. 277, 4925–4931 (2002).
Smart, E. J., De Rose, R. A. & Farber, S. A. Annexin 2–caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc. Natl Acad. Sci. USA 101, 3450–3455 (2004). Describes the involvement of an annexin-A2–caveolin-1 complex in intestinal sterol transport.
Raynal, P. & Pollard, H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1197, 63–93 (1994). A comprehensive annexin review that summarizes, among other things, their biochemical properties — in particular, their Ca2+- and phospholipid-binding affinities.
Berendes, R., Voges, D., Demange, P., Huber, R. & Burger, A. Structure–function analysis of the ion channel selectivity filter in human annexin V. Science 262, 427–430 (1993).
Demange, P. et al. Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem. Sci. 19, 272–276 (1994).
Nilius, B. et al. Annexin II modulates volume-activated chloride currents in vascular endothelial cells. J. Biol. Chem. 271, 30631–30636 (1996).
Okuse, K. et al. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 417, 653–656 (2002).
Girard, C. et al. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 21, 4439–4448 (2002).
van de Graaf, S. F. et al. Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10–annexin 2 complex. EMBO J. 22, 1478–1487 (2003).
Flower, R. J. & Rothwell, N. J. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 15, 71–76 (1994).
John, C. D. et al. Annexin 1 and the regulation of endocrine function. Trends Endocrinol. Metab. 15, 103–109 (2004).
Perretti, M. & Gavins, F. N. Annexin 1: an endogenous anti-inflammatory protein. News Physiol. Sci. 18, 60–64 (2003).
Perretti, M. & Flower, R. J. Annexin 1 and the biology of the neutrophil. J. Leukoc. Biol. 76, 25–29 (2004). An excellent review by two key investigators who work on the anti-inflammatory actions of annexin A1.
Walther, A., Riehemann, K. & Gerke, V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell 5, 831–840 (2000). This paper shows for the first time that annexin A1 functions as an endogenous ligand for FPR.
Perretti, M., Getting, S. J., Solito, E., Murphy, P. M. & Gao, J. L. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 158, 1969–1973 (2001).
Perretti, M. et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nature Med. 8, 1296–1302 (2002).
Ernst, S. et al. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J. Immunol. 172, 7669–7676 (2004). References 108 and 109 show that annexin A1 also functions as an agonist of the FPR-like receptors FPRL1 and FPRL2.
Prossnitz, E. R. & Ye, R. D. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol. Ther. 74, 73–102 (1997).
Gavins, F. N., Yona, S., Kamal, A. M., Flower, R. J. & Perretti, M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood 101, 4140–4147 (2003).
Hannon, R. et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 17, 253–255 (2003). Reveals aberrant inflammatory reactions and an elevated resistance to glucocorticoid treatment in annexin A1-null mice.
Yang, Y. H. et al. Modulation of inflammation and response to dexamethasone by annexin-1 in antigen-induced arthritis. Arthritis Rheum. 50, 976–984 (2004).
Rescher, U., Danielczyk, A., Markoff, A. & Gerke, V. Functional activation of the formyl peptide receptor by a new endogenous ligand in human lung A549 cells. J. Immunol. 169, 1500–1504 (2002).
Solito, E. et al. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J. 17, 1544–1546 (2003).
Arur, S. et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587–598 (2003).
Oh, P. et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429, 629–635 (2004).
Kim, J. & Hajjar, K. A. Annexin II: a plasminogen-plasminogen activator co-receptor. Front. Biosci. 7, d341–d348 (2002).
Ling, Q. et al. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 113, 38–48 (2004). Uses gene ablation in mice to show that annexin A2 participates in fibrinolysis and neoangiogenesis.
Sullivan, D. M., Wehr, N. B., Fergusson, M. M., Levine, R. L. & Finkel, T. Identification of oxidant-sensitive proteins: TNF-α induces protein glutathiolation. Biochemistry 39, 11121–11128 (2000).
Rowan, W. H. III, Sun, P. & Liu, L. Nitration of annexin II tetramer. Biochemistry 41, 1409–1420 (2002).
Hajjar, K. A. & Jacovina, A. T. Modulation of annexin II by homocysteine: implications for atherothrombosis. J. Investig. Med. 46, 364–369 (1998).
Ghitescu, L. D., Gugliucci, A. & Dumas, F. Actin and annexins I and II are among the main endothelial plasmalemma-associated proteins forming early glucose adducts in experimental diabetes. Diabetes 50, 1666–1674 (2001).
Rand, J. H. Antiphospholipid antibody-mediated disruption of the annexin V antithrombotic shield: a thrombogenetic mechanism for the antiphospholipid syndrome. J. Autoimmun. 15, 107–111 (2000).
Kretsinger, R. H. Structure and evolution of calcium modulated proteins. CRC Crit. Rev. Biochem. 8, 119–174 (1980).
Jahn, R., Lang, T. & Sudhof, T. C. Membrane fusion. Cell 112, 519–533 (2003).
Huber, R. et al. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J. Mol. Biol. 223, 683–704 (1992).
Burger, A. et al. The crystal structure and ion channel activity of human annexin II, a peripheral membrane protein. J. Mol. Biol. 257, 839–847 (1996).
Sopkova-de Oliveira Santos, J. et al. S100 protein–annexin interactions: a model of the (Anx2–p11)2 heterotetramer complex. Biochim. Biophys. Acta 1498, 181–191 (2000).
Oling, F., Bergsma-Schutter, W. & Brisson, A. Trimers, dimers of trimers, and trimers of trimers are common building blocks of annexin a5 two-dimensional crystals. J. Struct. Biol. 133, 55–63 (2001).
Acknowledgements
We thank our colleagues who provided unpublished information and materials that were used in the figures, and apologize to all those researchers whose work could not be discussed owing to space limitations. Work in the authors' laboratories is supported by: the Deutsche Forschungsgemeinschaft, the Interdisciplinary Center for Clinical Research of the Münster Medical School, and the European Union (V.G.); the National Institutes of Health (C.E.C.); and the Wellcome Trust, the Medical Research Council and Fight for Sight (S.E.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Prosite
Swiss-Prot
FURTHER INFORMATION
Glossary
- HYDRA ANNEXIN
-
Annexin B12 is the predominant annexin in the freshwater cnidarian Hydra vulgaris, and has been the prototype for biophysical studies on annexin insertion into phospholipid bilayers.
- EF-HAND SUPERFAMILY
-
The largest family of Ca2+-binding proteins, which is exemplified by calmodulin. The family members share a structural helix–loop–helix motif — the EF hand — that forms the Ca2+-binding site.
- S100 PROTEIN
-
A family of 1014–kDa, EF-hand-containing Ca2+-binding proteins, which transmit Ca2+-dependent cell-regulatory signals.
- PLECKSTRIN-HOMOLOGY-DOMAIN PROTEIN
-
Proteins that contain a pleckstrin-homology domain, which is a conserved motif that is most frequently associated with binding to inositol phospholipids.
- LIPID MICRODOMAIN
-
A localized membrane region that differs from the surrounding membrane in its lipid composition and order.
- LIPID RAFT
-
Lateral lipid aggregates that are rich in cholesterol and sphingolipids, and are thought to occur in cellular membranes. These lipid microdomains are resistant to solubilization by non-ionic detergents and probably resemble the liquid-ordered domains that are found in model membranes.
- CYTOKINESIS
-
The final stage of the cell-division cycle, in which two daughter cells become separated by the central spindle.
- MIDBODY
-
A dense protein matrix that forms at the midpoint of the central spindle during cytokinesis. Midbody proteins, of which annexin A11 is one, are required for cleavage-furrow formation and the final separation of daughter cells by abscission.
- P6 OR P3
-
Space groups that define crystal lattices with sixfold and threefold axes of symmetry, respectively.
- NUCLEOPLASM
-
The part of the nucleus that is contained by, but is distinct from, the nuclear envelope.
- HYDROXYAPATITE
-
A crystalline form of calcium phosphate that is present in the matrix of bone.
- CHROMAFFIN GRANULES
-
The secretory vesicles of the adrenal medulla. They contain noradrenaline or adrenaline, a number of biologically active peptides and high concentrations of ATP and ascorbic acid. The name is derived from the histological observation that the vesicles are readily stained by chromium salts.
- ARACHIDONIC ACID
-
A highly unsaturated, long-chain fatty acid (20 carbon atoms: 4 double bonds) that is often found at the sn-2 position of the glycerol backbone of membrane phospholipids. It is typically released by phospholipase action in stimulated cells, which allows it to function as a membrane fusogen or as the precursor of active signalling molecules such as the prostaglandins and leukotrienes.
- SNARE PROTEINS
-
(soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptor proteins). Integral membrane proteins in vesicle or cell-surface membranes that interact with one another during membrane fusion. The name is derived from the role of these proteins as receptors for a cytosolic protein, NSF, that is essential for organelle trafficking steps that involve membrane fusion.
- NON-ERYTHROID SPECTRIN
-
An isoform of spectrin that is sometimes also called fodrin and is expressed in cells other than erythrocytes.
- GLUCOCORTICOIDS
-
A class of steroid hormones with a potent anti-inflammatory activity.
- NEUTROPHIL EXTRAVASATION
-
The processs by which neutrophils (polymorphonuclear leukocytes) leave a blood vessel.
- PERITONITIS
-
Inflammation of the peritoneum (the membrane that lines the abdominal cavity and digestive organs of vertebrates).
- ACUTE-PHASE REACTION
-
The defense reaction of an organism to infectious or toxic agents, which helps to restrict organ damage through the cytokine-induced production of protective acute-phase proteins such as complement-reactive and serum-amyloid protein.
- JURKAT T-LYMPHOCYTES
-
A commonly used cell line that is derived from an acute lymphoblastic leukaemia of T-cell origin.
Rights and permissions
About this article
Cite this article
Gerke, V., Creutz, C. & Moss, S. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6, 449–461 (2005). https://doi.org/10.1038/nrm1661
Issue Date:
DOI: https://doi.org/10.1038/nrm1661
This article is cited by
-
Comprehensive analysis of annexin gene family and its expression in response to branching architecture and salt stress in crape myrtle
BMC Plant Biology (2024)
-
Potential prognostic and therapeutic value of ANXA8 in renal cell carcinoma: based on the comprehensive analysis of annexins family
BMC Cancer (2023)
-
Analysis of the potential biological mechanisms of diosmin against renal fibrosis based on network pharmacology and molecular docking approach
BMC Complementary Medicine and Therapies (2023)
-
ANXA6/TRPV2 axis promotes lymphatic metastasis in head and neck squamous cell carcinoma by inducing autophagy
Experimental Hematology & Oncology (2023)
-
Molecular characterization and functional implications on mouse peripheral blood mononuclear cells of annexin proteins from Echinococcus granulosus sensu lato
Parasites & Vectors (2023)