Key Points
-
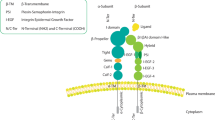
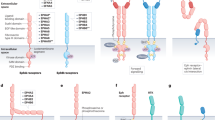
Dysregulated combined signalling between integrins and receptor tyrosine kinases (RTKs) promotes the disruption of adherens junctions at the onset of carcinoma invasion. Src-family kinases (SFKs) induce the expression of SNAIL/SLUG transcription factors, which repress transcription of the E-cadherin gene, and also promote — presumably through Hakai — endocytosis of E-cadherin protein. Integrin-linked kinase also promotes transcriptional repression of E-cadherin.
-
The integrins cooperate with RTKs to activate pro-migratory signalling pathways. Whereas focal adhesion kinase (FAK) signalling to Src induces the disassembly of focal adhesions at the rear of the cell, Rho-family GTPases coordinate the changes in the actin cytoskeleton that are necessary to anchor the leading edge of the cell to the matrix and propel the cell forward. The mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase (ERK) and Jun amino-terminal kinase (JNK) contribute to cell migration by phosphorylating various cytoskeletal signalling molecules and by promoting AP-1-dependent transcription.
-
The integrins facilitate matrix remodelling and the invasion of tumour cells through the recruitment of matrix-degrading proteases.
-
Tumour cells tend to upregulate or to maintain the expression of integrins that cooperate with RTKs to promote tumour progression, whereas they tend to lose the expression of integrins that exert the opposite effect.
-
Integrins have both adhesive and signalling roles during tumour angiogenesis. Various integrin–RTK pairs are likely to control angiogenesis — this will depend on the angiogenic stimulus, the tissue and the stage of angiogenesis. Integrins are targeted by activators as well as by inhibitors of angiogenesis. Joint integrin–RTK signalling controls the invasion of endothelial cells during angiogenesis, which implies that the invasion of tumour cells and tumour angiogenesis might be regulated by similar signalling mechanisms.
-
Integrins mediate the formation of microemboli, which are composed of tumour cells, platelets and leukocytes. They also facilitate the adhesion of tumour cells to the endothelium, which therefore promotes their docking and extravasation at a metastatic site.
-
Although cancer cells do not rely heavily on adhesion to the matrix for their survival and proliferation, dysregulated integrin–RTK signalling enhances the survival of cancer cells and the growth of primary tumours.
Abstract
During progression from tumour growth to metastasis, specific integrin signals enable cancer cells to detach from neighbouring cells, re-orientate their polarity during migration, and survive and proliferate in foreign microenvironments. There is increasing evidence that certain integrins associate with receptor tyrosine kinases (RTKs) to activate signalling pathways that are necessary for tumour invasion and metastasis. The effect of these integrins might be especially important in cancer cells that have activating mutations, or amplifications, of the genes that encode these RTKs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Giancotti, F. G. & Ruoslahti, E. Integrin signaling. Science 285, 1028–1032 (1999).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Giancotti, F. G. & Tarone, G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19, 173–206 (2003).
Miranti, C. K. & Brugge, J. S. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biol. 4, E83–E90 (2002).
Schwartz, M. A. & Ginsberg, M. H. Networks and crosstalk: integrin signalling spreads. Nature Cell Biol. 4, E65–E68 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Bissell, M. J. & Radisky, D. Putting tumours in context. Nature Rev. Cancer 1, 46–54 (2001).
Wiseman, B. S. & Werb, Z. Stromal effects on mammary gland development and breast cancer. Science 296, 1046–1049 (2002).
Hynes, R. O. Metastatic potential: generic predisposition of the primary tumor or rare, metastatic variants — or both? Cell 113, 821–823 (2003).
Liotta, L. A., Steeg, P. S. & Stetler-Stevenson, W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64, 327–336 (1991).
McDonald, D. M. & Baluk, P. Significance of blood vessel leakiness in cancer. Cancer Res. 62, 5381–5385 (2002).
Alitalo, K. & Carmeliet, P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1, 219–227 (2002).
Felding-Habermann, B. et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl Acad. Sci. USA 98, 1853–1858 (2001). Shows that integrin activation facilitates the adhesion of tumour cells to platelets in the circulation and, therefore, metastasis.
Borsig, L., Wong, R., Hynes, R. O., Varki, N. M. & Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl Acad. Sci. USA 99, 2193–2198 (2002).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Eliceiri, B. P. & Cheresh, D. A. Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 13, 563–568 (2001).
Hynes, R. O. A reevaluation of integrins as regulators of angiogenesis. Nature Med. 8, 918–921 (2002).
Mercurio, A. M. & Rabinovitz, I. Towards a mechanistic understanding of tumor invasion — lessons from the α6β4 integrin. Semin. Cancer Biol. 11, 129–141 (2001).
Breuss, J. M. et al. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108, 2241–2251 (1995).
Gladson, C. L. & Cheresh, D. A. Glioblastoma expression of vitronectin and the αvβ3 integrin: adhesion mechanism for transformed glial cells. J. Clin. Invest. 88, 1924–1932 (1991).
Albelda, S. M. et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 50, 6757–6764 (1990).
Zutter, M. M., Santoro, S. A., Staatz, W. D. & Tsung, Y. L. Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl Acad. Sci. USA 92, 7411–7415 (1995).
Plantefaber, L. C. & Hynes, R. O. Changes in integrin receptors on oncogenically transformed cells. Cell 56, 281–290 (1989).
Vleminckx, K., Vakaet, L. Jr., Mareel, M., Fiers, W. & van Roy, F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119 (1991).
Perl, A. K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (1998). Provides genetic evidence that loss of E-cadherin promotes malignant conversion of carcinoma.
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nature Rev. Mol. Cell Biol. 3, 155–166 (2002).
Weaver, V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997). Provides evidence that β 1 -integrin signalling contributes to the disruption of epithelial polarity in breast carcinoma cells.
Gimond, C. et al. Induction of cell scattering by expression of β1 integrins in β1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J. Cell Biol. 147, 1325–1340 (1999).
Sander, E. E. et al. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143, 1385–1398 (1998).
Fujita, Y. et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nature Cell Biol. 4, 222–231 (2002).
Avizienyte, E. et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nature Cell Biol. 4, 632–638 (2002). Identifies a role for FAK in the disruption of adherens junctions in colon cancer.
Novak, A. et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl Acad. Sci. USA 95, 4374–4379 (1998).
Tan, C. et al. Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene 20, 133–140 (2001).
Zhang, F. et al. Distinct ligand binding sites in integrin α3β1 regulate matrix adhesion and cell–cell contact. J. Cell Biol. 163, 177–188 (2003).
Siegel, P. M. & Massague, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nature Rev. Cancer 3, 807–821 (2003).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 29, 117–129 (2001).
Munger, J. S. et al. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Mu, D. et al. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157, 493–507 (2002).
Inaguma, Y. et al. Epithelial induction of stromal tenascin in the mouse mammary gland: from embryogenesis to carcinogenesis. Dev. Biol. 128, 245–255 (1988).
Agrez, M., Chen, A., Cone, R. I., Pytela, R. & Sheppard, D. The αvβ6 integrin promotes proliferation of colon carcinoma cells through a unique region of the β6 cytoplasmic domain. J. Cell Biol. 127, 547–556 (1994).
Webb, D. J., Parsons, J. T. & Horwitz, A. F. Adhesion assembly, disassembly and turnover in migrating cells — over and over and over again. Nature Cell Biol. 4, E97–E100 (2002).
Ilic, D. et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 (1995). Shows that FAK has a key role in cell migration.
Gabarra-Niecko, V., Schaller, M. D. & Dunty, J. M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 22, 359–374 (2003).
Sieg, D. J. et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nature Cell Biol. 2, 249–256 (2000).
Cary, L. A., Han, D. C., Polte, T. R., Hanks, S. K. & Guan, J. L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140, 211–221 (1998).
Klemke, R. L. et al. CAS/Crk coupling serves as a 'molecular switch' for induction of cell migration. J. Cell Biol. 140, 961–972 (1998). References 47 and 48 show that CAS and Crk function downstream of FAK in pro-migratory signalling.
Chen, R. et al. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nature Cell Biol. 3, 439–444 (2001).
Webb, D. J. et al. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 6, 154–161 (2004).
Gu, J. et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J. Cell Biol. 146, 389–403 (1999).
Klemke, R. L. et al. Regulation of cell motility by mitogen-activated protein kinase. J. Cell. Biol. 137, 481–492 (1997).
Huang, C., Rajfur, Z., Borchers, C., Schaller, M. D. & Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 424, 219–223 (2003).
Ishibe, S., Joly, D., Zhu, X. & Cantley, L. G. Phosphorylation-dependent paxillin–ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell 12, 1275–1285 (2003).
Li, G. et al. c-Jun is essential for organization of the epidermal leading edge. Dev. Cell 4, 865–877 (2003).
Raftopoulou, M. & Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 (2004).
Keely, P. J., Westwick, J. K., Whitehead, I. P., Der, C. J. & Parise, L. V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390, 632–636 (1997).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Edwards, D. C., Sanders, L. C., Bokoch, G. M. & Gill, G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature Cell Biol. 1, 253–259 (1999).
Etienne-Manneville, S. & Hall, A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell 106, 489–498 (2001). Shows that integrin signalling at the leading edge regulates, through Cdc42 and PAR6–PKCζ, cell polarity during migration.
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nature Cell Biol. 1, 136–143 (1999).
Kimura, K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 (1996).
Sahai, E. & Marshall, C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nature Cell Biol. 5, 711–719 (2003). Shows a key role for Rho–ROCK signalling during amoeboid invasion of tumour cells.
Itoh, K. et al. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Med. 5, 221–225 (1999).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000). Identifies, through global gene-expression analysis, RhoC as an important determinant for melanoma metastasis.
Sternlicht, M. D. & Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516 (2001).
Hsu, M. Y. et al. Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am. J. Pathol. 153, 1435–1442 (1998). Provides evidence that overexpression of α v β 3 in melanoma cells promotes the transition from the radial, non-invasive to the vertical, invasive growth phase.
Brooks, P. C. et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3 . Cell 85, 683–693 (1996). Shows that the α v β 3 integrin promotes the invasion of tumour cells by recruiting active MMP2 to the cell surface.
Brooks, P. C., Silletti, S., von Schalscha, T. L., Friedlander, M. & Cheresh, D. A. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 92, 391–400 (1998).
Chapman, H. A. & Wei, Y. Protease crosstalk with integrins: the urokinase receptor paradigm. Thromb. Haemost. 86, 124–129 (2001).
Blasi, F. & Carmeliet, P. uPAR: a versatile signalling orchestrator. Nature Rev. Mol. Cell Biol. 3, 932–943 (2002).
Hauck, C. R., Hsia, D. A., Puente, X. S., Cheresh, D. A. & Schlaepfer, D. D. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 21, 6289–6302 (2002).
Hsia, D. A. et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160, 753–767 (2003).
Liu, D., Aguirre-Ghiso, J., Estrada, Y. & Ossowski, L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1, 445–457 (2002). Shows that uPAR enhances joint integrin–RTK signalling during carcinoma growth.
Kjoller, L. & Hall, A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J. Cell Biol. 152, 1145–1157 (2001).
Patarroyo, M., Tryggvason, K. & Virtanen, I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 12, 197–207 (2002).
Koshikawa, N., Giannelli, G., Cirulli, V., Miyazaki, K. & Quaranta, V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 148, 615–624 (2000).
Giannelli, G., Falk-Marzillier, J., Schiraldi, O., Stetler-Stevenson, W. G. & Quaranta, V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277, 225–228 (1997).
Xu, J. et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 154, 1069–1080 (2001).
Kajiji, S., Tamura, R. N. & Quaranta, V. A novel integrin (αEβ4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 8, 673–680 (1989).
Mainiero, F. et al. The coupling of α6β4 integrin to Ras–MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16, 2365–2375 (1997).
Dajee, M. et al. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421, 639–643 (2003).
Shaw, L. M., Rabinovitz, I., Wang, H. H., Toker, A. & Mercurio, A. M. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 91, 949–960 (1997). Shows that α 6 β 4 promotes invasion of tumour cells, and implies that it does so through activation of PI3K.
Trusolino, L., Bertotti, A. & Comoglio, P. M. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell 107, 643–654 (2001).
Weaver, V. M. et al. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2, 205–216 (2002). Shows a role for α 6 β 4 integrin in conferring resistance to apoptotic stimuli in breast carcinoma cells.
Zahir, N. et al. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J. Cell Biol. 163, 1397–1407 (2003).
Mariotti, A. et al. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 155, 447–458 (2001). References 84 and 87 provide evidence that RTK-mediated phosphorylation of the β 4 cytoplasmic tail converts α 6 β 4 from a pro-adhesive to a pro-migratory receptor, through disruption of hemidesmosomes and activation of SHC and PI3K.
Falcioni, R. et al. α6β4 and α6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell Res. 236, 76–85 (1997).
Santoro, M. M., Gaudino, G. & Marchisio, P. C. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell 5, 257–271 (2003).
Owens, D. M. & Watt, F. M. Influence of β1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: α3β1, but not α2β1, suppresses malignant conversion. Cancer Res. 61, 5248–5254 (2001).
Wary, K. K., Mainiero, F., Isakoff, S. J., Marcantonio, E. E. & Giancotti, F. G. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733–743 (1996).
Ivaska, J. et al. Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J. Cell Biol. 147, 401–416 (1999).
Ellinger-Ziegelbauer, H., Kelly, K. & Siebenlist, U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol. Cell. Biol. 19, 3857–3868 (1999).
Chan, B. M., Matsuura, N., Takada, Y., Zetter, B. R. & Hemler, M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science 251, 1600–1602 (1991).
Wang, H. et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell. Biol. 164, 935–941 (2004).
Byzova, T. V. et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6, 851–860 (2000).
Klein, S., Bikfalvi, A., Birkenmeier, T. M., Giancotti, F. G. & Rifkin, D. B. Integrin regulation by endogenous expression of 18-kDa fibroblast growth factor-2. J. Biol. Chem. 271, 22583–22590 (1996).
Rehn, M. et al. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl Acad. Sci. USA 98, 1024–1029 (2001).
Hamano, Y. et al. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αvβ1 integrin. Cancer Cell 3, 589–601 (2003).
Sheppard, D. Endothelial integrins and angiogenesis: not so simple anymore. J. Clin. Invest. 110, 913–914 (2002).
Frisch, S. M. & Ruoslahti, E. Integrins and anoikis. Curr. Opin. Cell Biol. 9, 701–706 (1997).
Frisch, S. M., Vuori, K., Ruoslahti, E. & Chan-Hui, P. Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 134, 793–799 (1996).
Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H. & Downward, J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997). References 102 and 103 provide evidence that FAK promotes cell survival through signalling from PI3K to AKT/PKB.
Zhang, Z., Vuori, K., Reed, J. C. & Ruoslahti, E. The α5β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl Acad. Sci. USA 92, 6161–6165 (1995).
Reginato, M. J. et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nature Cell Biol. 5, 733–740 (2003).
Butt, A. J., Firth, S. M. & Baxter, R. C. The IGF axis and programmed cell death. Immunol. Cell Biol. 77, 256–262 (1999).
Lotem, J. & Sachs, L. Control of apoptosis in hematopoiesis and leukemia by cytokines, tumor suppressor and oncogenes. Leukemia 10, 925–931 (1996).
Tamura, M. et al. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 274, 20693–20703 (1999).
Fridman, J. S. & Lowe, S. W. Control of apoptosis by p53. Oncogene 22, 9030–9040 (2003).
Ilic, D. et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143, 547–560 (1998).
Frisch, S. M. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr. Biol. 9, 1047–1049 (1999).
Rytomaa, M., Martins, L. M. & Downward, J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr. Biol. 9, 1043–1046 (1999).
Aoudjit, F. & Vuori, K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152, 633–643 (2001).
Pitti, R. M. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396, 699–703 (1998).
Stupack, D. G., Puente, X. S., Boutsaboualoy, S., Storgard, C. M. & Cheresh, D. A. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155, 459–470 (2001).
Plath, T. et al. A novel function for the tumor suppressor p16INK4a: induction of anoikis via upregulation of the α5β1 fibronectin receptor. J. Cell Biol. 150, 1467–1478 (2000). Provides a mechanism by which loss of tumor suppressor p16INK4a contributes to resistance to anoikis in tumour cells.
Bachelder, R. E., Marchetti, A., Falcioni, R., Soddu, S. & Mercurio, A. M. Activation of p53 function in carcinoma cells by the α6β4 integrin. J. Biol. Chem. 274, 20733–20737 (1999).
Camerer, E. et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104, 397–401 (2004).
Abdel-Ghany, M., Cheng, H. C., Elble, R. C. & Pauli, B. U. Focal adhesion kinase activated by β4 integrin ligation to mCLCA1 mediates early metastatic growth. J. Biol. Chem. 277, 34391–34400 (2002).
Voura, E. B., Ramjeesingh, R. A., Montgomery, A. M. & Siu, C. H. Involvement of integrin αvβ3 and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol. Biol. Cell 12, 2699–2710 (2001).
Parsons, J. T. & Parsons, S. J. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 9, 187–192 (1997).
Schlaepfer, D. D. & Hunter, T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8, 151–157 (1998).
Cary, L. A., Han, D. C. & Guan, J. L. Integrin-mediated signal transduction pathways. Histol. Histopathol. 14, 1001–1009 (1999).
Wary, K. K., Mariotti, A., Zurzolo, C. & Giancotti, F. G. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625–634 (1998).
Arias-Salgado, E. G. et al. Src kinase activation by direct interaction with the integrin βcytoplasmic domain. Proc. Natl Acad. Sci. USA 100, 13298–13302 (2003).
Gagnoux-Palacios, L. et al. Compartmentalization of integrin α6β4 signaling in lipid rafts. J. Cell Biol. 162, 1189–1196 (2003).
Mainiero, F. et al. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 14, 4470–4481 (1995).
Shaw, L. M. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the α6β4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell Biol. 21, 5082–5093 (2001).
Gambaletta, D. et al. Cooperative signaling between α6β4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem. 275, 10604–10610 (2000).
Schneller, M., Vuori, K. & Ruoslahti, E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 16, 5600–5607 (1997).
Dai, C. et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 15, 1913–1925 (2001).
Acknowledgements
Research in the authors' laboratory is supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Swiss-Prot
Glossary
- INTERSTITIAL MATRIX
-
The extracellular matrix that resides in connective tissues.
- TYPE-I TRANSMEMBRANE PROTEIN
-
A protein that contains a single membrane-spanning domain, with the carboxyl terminus orientated towards the cytoplasm and the amino terminus orientated towards the lumen of membrane compartments or in an extracellular direction.
- SRC FAMILY KINASES
-
(SFKs). Kinases that belong to the Src family of tyrosine kinases, the largest of the non-receptor-tyrosine-kinase families. SFKs include Src, Yes, Fyn, Lck, Lyn, Blk, Hck, Fgr and Yrk.
- PALMITOYLATED
-
The post-translational modification of a protein with palmitic acid.
- LIPID RAFTS
-
Membrane microdomains that are enriched in cholesterol, sphingolipids and lipid-modified proteins such as GPI-linked proteins and palmitoylated proteins. These microdomains often function as platforms for signalling events.
- TIGHT JUNCTION
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells. Tight junctions regulate paracellular flux, and contribute to the maintenance of cell polarity by stopping molecules from diffusing in the plane of the membrane.
- PLATELETS
-
The smallest blood cells, which are important in haemostasis and blood coagulation.
- GUANINE NUCLEOTIDE-EXCHANGE FACTOR
-
(GEF). A protein that facilitates the exchange of GDP (guanine diphosphate) for GTP (guanine triphosphate) in the nucleotide-binding pocket of a GTP-binding protein.
- CARCINOMA
-
A malignant tumour that originates from epithelial tissue.
- ADENOMA
-
A benign tumour that arises from glandular epithelium.
- HEMIDESMOSOMES
-
Adhesion complexes that connect intracellular keratin filaments with extracellular matrix in the basement membrane, and thereby mediate stable cell–matrix adhesion. These multiprotein complexes are assembled by integrin α6β4, BP180 and plectins.
- EPITHELIAL–MESENCHYMAL TRANSITION
-
(EMT). The transformation of an epithelial cell into a mesenchymal cell with migratory and invasive properties.
- ADHERENS JUNCTION
-
A cell–cell adhesion complex that contains cadherins and catenins that are attached to cytoplasmic actin filaments.
- MATRIGEL
-
The extracellular matrix secreted by the Engelbrecht–Holm–Swarm mouse sarcoma cell line. It contains laminin, collagen IV, nidogen/entactin and proteoglycans, and so resembles the basement membrane.
- ACINI
-
Differentiated epithelial structures in which a single layer of polarized epithelial cells encompasses a hollow lumen.
- RHO-FAMILY GTPASES
-
Ras-related GTPases that are involved in controlling the dynamics of the actin cytoskeleton.
- E3 UBIQUITIN PROTEIN LIGASE
-
The third enzyme in a series — the first two are designated E1 and E2 — that are responsible for ubiquitylation of target proteins. E3 enzymes provide platforms for binding E2 enzymes and specific substrates, thereby coordinating ubiquitylation of the selected substrates.
- FILOPODIA
-
Long, thin protrusions at the periphery of cells and growth cones. They are composed of F-actin bundles.
- FOCAL ADHESION
-
An integrin-mediated cell–substrate adhesion structure that anchors the ends of actin filaments (stress fibres) and mediates strong attachments to substrates. It also functions as an integrin signalling platform.
- LAMELLIPODIA
-
Flattened, sheet-like structures — which are composed of a crosslinked F-actin meshwork — that project from the surface of a cell. They are often associated with cell migration.
- STRESS FIBRE
-
A bundle of parallel filaments that contain F-actin and other contractile molecules. It often stretches between cell attachments as if under stress.
- ARP2/3 PROTEIN COMPLEX
-
A complex that consists of two actin-related proteins, ARP2 and ARP3, along with five smaller proteins. When activated, the ARP2/3 complex binds to the side of an existing actin filament and nucleates the assembly of a new actin filament. The resulting branch structure is Y-shaped.
- RGD SEQUENCE
-
The primary adhesive motif in many extracellular-matrix molecules, which contains the amino-acid triplet Arg-Gly-Asp.
- TRANSIT-AMPLIFYING CELLS
-
Progenitor cells that are able to divide only 3–5 times before all of their daughters terminally differentiate.
- DOMINANT-NEGATIVE
-
A defective protein that inhibits the function of normal proteins often by competing with normal proteins for interacting molecules through retained interaction capabilities.
- ANOIKIS
-
Induction of programmed cell death by detachment of cells from the extracellular matrix.
- SELECTINS
-
A family of adhesion receptors that bind to the carbohydrate groups of heavily glycosylated counter-receptors through their lectin domain. Selectins mediate the adhesion of leukocytes to endothelium.
Rights and permissions
About this article
Cite this article
Guo, W., Giancotti, F. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5, 816–826 (2004). https://doi.org/10.1038/nrm1490
Issue Date:
DOI: https://doi.org/10.1038/nrm1490
This article is cited by
-
ITGB1 and DDR activation as novel mediators in acquired resistance to osimertinib and MEK inhibitors in EGFR-mutant NSCLC
Scientific Reports (2024)
-
Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities
Cell Communication and Signaling (2023)
-
Patient-derived xenograft models in cancer therapy: technologies and applications
Signal Transduction and Targeted Therapy (2023)
-
Molecular basis for the recognition of 24-(S)-hydroxycholesterol by integrin αvβ3
Scientific Reports (2023)
-
Bovine holo-lactoferrin inhibits migration and invasion in MDA-MB-231 breast cancer cells
Molecular Biology Reports (2023)