Key Points
-
During an infection, T cells can differentiate into multiple types of effector and memory T cell that help to mediate pathogen clearance and provide long-term protective immunity.
-
Hypothetical models — known as the separate-precursor, decreasing-potential, signal-strength and asymmetric cell fate models — help to explain the generation of heterogeneous effector and memory T cells.
-
The strength and duration of signals 1, 2 and 3 (mediated by antigen, co-stimulatory molecules and cytokines, respectively) can influence effector T cell fate decisions.
-
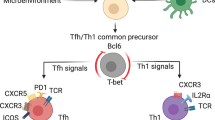
The expression or activity of counter-regulatory transcription factors functions in a graded manner to regulate effector and memory T cell differentiation. Such transcription factor pairs include: T-bet and eomesodermin; B lymphocyte-induced maturation protein 1 (BLIMP1) and B cell lymphoma 6 (BCL-6); inhibitor of DNA binding 2 (ID2) and ID3; and signal transducer and activator of transcription 3 (STAT3) and STAT4.
-
Cellular metabolic fitness and metabolic signalling pathways — mainly the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK)–forkhead box O (FOXO) pathways — regulate T cell differentiation, effector function and lifespan.
-
Cytokine signalling and transcriptional programmes control the progressive differentiation or functional maturation of memory T cells during the effector-to-memory cell transition.
-
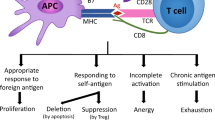
During chronic infection, phenotypic, functional and transcriptomic diversification of CD8+ T cells occurs over multiple rounds of stimulation.
Abstract
During an infection, T cells can differentiate into multiple types of effector and memory T cells, which help to mediate pathogen clearance and provide long-term protective immunity. These cells can vary in their phenotype, function and location, and in their long-term fate in terms of their ability to populate the memory T cell pool. Over the past decade, the signalling pathways and transcriptional programmes that regulate the formation of heterogeneous populations of effector and memory CD8+ T cells have started to be characterized, and this Review discusses the major advances in these areas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marshall, H. D. et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4+ cell properties during viral infection. Immunity 35, 633–646 (2011).
Pepper, M., Pagan, A. J., Igyarto, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011).
Roman, E. et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196, 957–968 (2002).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007). This study shows that pro-inflammatory cytokines (such as IL-12) induce the terminal differentiation of effector CD8+ T cells through the induction of increased T-bet expression.
Joshi, N. S. & Kaech, S. M. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 180, 1309–1315 (2008).
Sarkar, S. et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640 (2008).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000).
Croom, H. A. et al. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur. J. Immunol. 41, 682–693 (2011).
Obar, J. J. et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 187, 4967–4978 (2011).
Masopust, D., Ha, S. J., Vezys, V. & Ahmed, R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 177, 831–839 (2006).
Nolz, J. C. & Harty, J. T. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity 34, 781–793 (2011).
Joshi, N. S. et al. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J. Immunol. 187, 4068–4076 (2011).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Jameson, S. C. & Masopust, D. Diversity in T cell memory: an embarrassment of riches. Immunity 31, 859–871 (2009).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Wherry, E. J. T cell exhaustion. Nature Immunol. 12, 492–499 (2011).
Virgin, H. W., Wherry, E. J. & Ahmed, R. Redefining chronic viral infection. Cell 138, 30–50 (2009).
Stemberger, C. et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity 27, 985–997 (2007).
Gerlach, C. et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J. Exp. Med. 207, 1235–1246 (2010). References 20 and 21 provide evidence that a single naive CD8+ T cell can give rise to both terminal effector and memory T cells.
Badovinac, V. P., Messingham, K. A., Jabbari, A., Haring, J. S. & Harty, J. T. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature Med. 11, 748–756 (2005).
Badovinac, V. P., Porter, B. B. & Harty, J. T. CD8+ T cell contraction is controlled by early inflammation. Nature Immunol. 5, 809–817 (2004).
D'Souza, W. N. & Hedrick, S. M. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J. Immunol. 177, 777–781 (2006).
Lanzavecchia, A. & Sallusto, F. Progressive differentiation and selection of the fittest in the immune response. Nature Rev. Immunol. 2, 982–987 (2002).
Chang, J. T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007). This paper illustrates that memory and effector T cell fates can arise from a single precursor T cell through asymmetric cell division.
Mousavi, S. F. et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J. Immunol. 181, 5990–6001 (2008).
Hendriks, J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 1, 433–440 (2000).
Hendriks, J. et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J. Immunol. 175, 1665–1676 (2005).
Williams, M. A. & Bevan, M. J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Pulle, G., Vidric, M. & Watts, T. H. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J. Immunol. 176, 2739–2748 (2006).
Salek-Ardakani, S. et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J. Clin. Invest. 121, 296–307 (2011).
Mescher, M. F. et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211, 81–92 (2006).
Curtsinger, J. M., Johnson, C. M. & Mescher, M. F. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171, 5165–5171 (2003).
Haring, J. S., Badovinac, V. P., Olson, M. R., Varga, S. M. & Harty, J. T. In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-γ receptor. J. Immunol. 175, 3117–3122 (2005).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010). References 37 and 38 indicate that IL-2-mediated signalling promotes the terminal differentiation of effector CD8+ T cells.
Whitmire, J. K., Eam, B., Benning, N. & Whitton, J. L. Direct interferon-γ signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 179, 1190–1197 (2007).
Whitmire, J. K., Tan, J. T. & Whitton, J. L. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 201, 1053–1059 (2005).
Bonilla, W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8 T cell responses. Science 335, 984–989 (2012).
Agarwal, P. et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J. Immunol. 183, 1695–1704 (2009).
Wherry, E. J., Puorro, K. A., Porgador, A. & Eisenlohr, L. C. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J. Immunol. 163, 3735–3745 (1999).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Kaech, S. M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2, 415–422 (2001).
Marzo, A. L. et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nature Immunol. 6, 793–799 (2005).
Cui, W., Joshi, N. S., Jiang, A. & Kaech, S. M. Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27, 2177–2187 (2009).
Badovinac, V. P. & Harty, J. T. Manipulating the rate of memory CD8+ T cell generation after acute infection. J. Immunol. 179, 53–63 (2007).
Prlic, M., Hernandez-Hoyos, G. & Bevan, M. J. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 203, 2135–2143 (2006).
Pearce, E. L. & Shen, H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 179, 2074–2081 (2007).
Keppler, S. J., Theil, K., Vucikuja, S. & Aichele, P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8+ T cells. Eur. J. Immunol. 39, 1774–1783 (2009).
Wiesel, M. et al. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur. J. Immunol. 42, 320–329 (2012).
Hu, J. K., Kagari, T., Clingan, J. M. & Matloubian, M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc. Natl Acad. Sci. USA 108, e118–e127 (2011).
Kurachi, M. et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 208, 1605–1620 (2011).
Kohlmeier, J. E. et al. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J. Exp. Med. 208, 1621–1634 (2011).
Jung, Y. W., Rutishauser, R. L., Joshi, N. S., Haberman, A. M. & Kaech, S. M. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J. Immunol. 185, 5315–5325 (2010).
Kastenmuller, W. et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol. 187, 3186–3197 (2011).
Foulds, K. E., Rotte, M. J. & Seder, R. A. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J. Immunol. 177, 2565–2574 (2006).
Sanjabi, S., Mosaheb, M. M. & Flavell, R. A. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31, 131–144 (2009).
Intlekofer, A. M. et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204, 2015–2021 (2007).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunol. 6, 1236–1244 (2005).
Banerjee, A. et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992 (2010).
Intlekofer, A. M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Takemoto, N., Intlekofer, A. M., Northrup, J. T., Wherry, E. J. & Reiner, S. L. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 177, 7515–7519 (2006).
Rao, R. R., Li, Q., Odunsi, K. & Shrikant, P. A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity 32, 67–78 (2010).
Cruz-Guilloty, F. et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59 (2009).
Rao, R. R., Li, Q., Gubbels Bupp, M. R. & Shrikant, P. A. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36, 374–387 (2012).
Zhou, X. et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 (2010).
Crotty, S., Johnston, R. J. & Schoenberger, S. P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunol. 11, 114–120 (2010).
Kwon, H. et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 31, 941–952 (2009).
Gong, D. & Malek, T. R. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J. Immunol. 178, 242–252 (2007).
Rutishauser, R. L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Kallies, A., Xin, A., Belz, G. T. & Nutt, S. L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009). References 72–74 collectively demonstrate that BLIMP1 promotes the terminal differentiation or exhaustion of CD8+ T cells in acute and chronic viral infections.
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21–interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Ichii, H. et al. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int. Immunol. 19, 427–433 (2007).
Ichii, H. et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nature Immunol. 3, 558–563 (2002).
Ichii, H., Sakamoto, A., Kuroda, Y. & Tokuhisa, T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J. Immunol. 173, 883–891 (2004).
Oestreich, K. J., Huang, A. C. & Weinmann, A. S. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 208, 1001–1013 (2011).
Oestreich, K. J., Mohn, S. E. & Weinmann, A. S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature Immunol. 13, 405–411 (2012).
Ji, Y. et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nature Immunol. 12, 1230–1237 (2011).
Cannarile, M. A. et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nature Immunol. 7, 1317–1325 (2006).
Yang, C. Y. et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature Immunol. 12, 1221–1229 (2011).
O'Shea, J. J. & Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550 (2012).
Gough, D. J. et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324, 1713–1716 (2009).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009).
Choi, Y. S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Gil, M. P., Salomon, R., Louten, J. & Biron, C. A. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 107, 987–993 (2006).
Nguyen, K. B. et al. Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science 297, 2063–2066 (2002).
Xiao, Z., Casey, K. A., Jameson, S. C., Curtsinger, J. M. & Mescher, M. F. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J. Immunol. 182, 2786–2794 (2009).
Lin, J. X. et al. Critical role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity 36, 586–599 (2012).
Palmer, D. C. & Restifo, N. P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30, 592–602 (2009).
Seki, Y. et al. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl Acad. Sci. USA 99, 13003–13008 (2002).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunol. 12, 295–303 (2011).
Siegel, A. M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011). This paper, together with reference 75, demonstrates that cytokine signalling through the STAT3 pathway is required for the functional maturation and maintenance of memory CD8+ T cells.
Yang, X. P. et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature Immunol. 12, 247–254 (2011).
O'Shea, J. J., Lahesmaa, R., Vahedi, G., Laurence, A. & Kanno, Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nature Rev. Immunol. 11, 239–250 (2011).
Weng, N. P., Araki, Y. & Subedi, K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nature Rev. Immunol. 12, 306–315 (2012).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nature Rev. Immunol. 12, 325–338 (2012).
Pearce, E. L. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 22, 314–320 (2010).
Michalek, R. D. & Rathmell, J. C. The metabolic life and times of a T-cell. Immunol. Rev. 236, 190–202 (2010).
Finlay, D. & Cantrell, D. A. Metabolism, migration and memory in cytotoxic T cells. Nature Rev. Immunol. 11, 109–117 (2011).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009). References 103 and 104 were the first studies to describe the role of metabolic regulation in the differentiation of memory and effector CD8+ T cells.
Sinclair, L. V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 9, 513–521 (2008).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Kerdiles, Y. M. et al. Foxo transcription factors control regulatory T cell development and function. Immunity 33, 890–904 (2010).
Macintyre, A. N. et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236 (2011).
Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
van Lier, R. A., ten Berge, I. J. & Gamadia, L. E. Human CD8+ T-cell differentiation in response to viruses. Nature Rev. Immunol. 3, 931–939 (2003).
Xue, H. H. & Zhao, D. M. Regulation of mature T cell responses by the Wnt signaling pathway. Ann. NY Acad. Sci. 1247, 16–33 (2012).
Zhao, D. M. et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 184, 1191–1199 (2010).
Jeannet, G. et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl Acad. Sci. USA 107, 9777–9782 (2010).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature Med. 15, 808–813 (2009).
Jabbari, A. & Harty, J. T. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 203, 919–932 (2006).
Wirth, T. C. et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 33, 128–140 (2010).
Wirth, T. C., Martin, M. D., Starbeck-Miller, G., Harty, J. T. & Badovinac, V. P. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur. J. Immunol. 41, 1321–1333 (2011).
Voehringer, D. et al. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167, 4838–4843 (2001).
Vezys, V. et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457, 196–199 (2009).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Mazo, I. B. et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 22, 259–270 (2005).
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
Sheridan, B. S. & Lefrancois, L. Regional and mucosal memory T cells. Nature Immunol. 12, 485–491 (2011).
Bouneaud, C., Garcia, Z., Kourilsky, P. & Pannetier, C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J. Exp. Med. 201, 579–590 (2005).
Wherry, E. J. & Ahmed, R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78, 5535–5545 (2004).
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 4, 225–234 (2003).
Marzo, A. L., Yagita, H. & Lefrancois, L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 179, 36–40 (2007).
Teijaro, J. R. et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).
Wakim, L. M., Woodward-Davis, A. & Bevan, M. J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA 107, 17872–17879 (2010).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Kao, C. et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nature Immunol. 12, 663–671 (2011).
Quigley, M. et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature Med. 16, 1147–1151 (2010).
Acknowledgements
We thank the members of the Kaech laboratory for helpful comments and discussions. This work was supported by grants to S.M.K. from the US National Institutes of Health (grants R01AI074699, RO1AI066232, R21AI097767 and R21AI081150) and from the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Type 1 responses
-
Coordinated immune responses that occur following viral or intracellular bacterial infection. They are usually characterized by the rapid induction of innate cytokines such as interleukin-12 and interferons and the development of T helper 1 (TH1) cells and cytotoxic T cells.
- T follicular helper cells
-
(TFH cells). A distinct subset of CD4+ helper T cells that are CXCR5hiPD1hi. These cells primarily migrate into germinal centres following immunization, where they regulate the development of antigen-specific B cell immune responses.
- Central memory T cells
-
(TCM cells). A subset of memory T cells that are normally CD62LhiCCR7hi and that home to the secondary lymphoid organs.
- Effector memory T cells
-
(TEM cells). A subset of memory T cells that are normally CD62LlowCCR7low and that reside in non-lymphoid tissues.
- Cellular barcoding
-
A tool for clonal analysis. Retroviral vectors with random sequences (that are referred to as 'barcodes') are transduced into progenitor cells. On integration, each vector introduces a unique, identifiable and heritable mark into the host cell genome, allowing the clonal progeny of each cell to be tracked over time.
- Asymmetric cell division
-
A process that produces two daughter cells with different cellular fates. This is in contrast to symmetric cell division, which gives rise to daughter cells of equivalent fates.
- T-box transcription factors
-
A family of transcription factors characterized by their homologous T-box DNA-binding domain.
- Mammalian target of rapamycin
-
(mTOR). A serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis and transcription. mTOR belongs to the phosphoinositide 3-kinase (PI3K)-related kinase protein family. The PI3K–AKT–mTOR pathway is activated by T cell receptor signalling and sustained by pro-inflammatory cytokines such as IL-2 and IL-12.
- WNT
-
A family of glycoproteins related to the Drosophila melanogaster protein Wingless, a ligand that regulates the temporal and spatial development of the embryo. WNT-mediated signalling has been shown to regulate cell fate determination, proliferation, adhesion, migration and polarity during development. In addition to their crucial role in embryogenesis, WNT proteins and their downstream signalling molecules have been implicated in tumorigenesis and have causative roles in human colon cancers. WNT signalling activates TCF and LEF family transcription factors by stabilizing their co-activator, β-catenin, and mobilizing this factor from the cytoplasm to the nucleus.
- E protein transcription factors
-
Key transcriptional regulators that control many aspects of lymphocyte development. E proteins bind as homodimers or heterodimers to DNA at their canonical E box sites, where they function as transcriptional activators or repressors. There are four E proteins in mammals, namely E47, E12, HEB and E2-2.
- JAK–STAT signalling
-
A signalling pathway that transmits information from cell-surface receptors for specific chemical signals outside the cell to gene promoters in the DNA in the cell nucleus, which causes DNA transcription and activity in the cell.
- Hyper-IgE syndrome
-
(Also known as Job's syndrome). A heterogeneous group of immune disorders caused by autosomal dominant mutation of STAT3. It is characterized by recurrent infections and very high concentrations of the serum antibody IgE.
- mTORC1
-
(mTOR complex 1). A complex composed of mTOR, regulatory associated protein of mTOR (RAPTOR), LST8 (also known as GβL), RAS40 and DEPTOR. This complex is characterized by the classic features of mTOR, in that it functions as a nutrient, energy and redox sensor and controls protein synthesis.
- mTORC2
-
(mTOR complex 2). A complex composed of mTOR, rapamycin-insensitive companion of mTOR (RICTOR), LST8 and SAPK-interacting protein 1 (SIN1). mTORC2 phosphorylates the serine/threonine protein kinase AKT at a serine residue (S473). mTORC2 has also been shown to function as an important regulator of the cytoskeleton.
- Hayflick limit
-
The number of times that a normal cell population will divide before it stops and enters a phase of senescence.
Rights and permissions
About this article
Cite this article
Kaech, S., Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12, 749–761 (2012). https://doi.org/10.1038/nri3307
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3307
This article is cited by
-
PD-1 signaling uncovers a pathogenic subset of T cells in inflammatory arthritis
Arthritis Research & Therapy (2024)
-
Activation of mucosal insulin receptor exacerbates intestinal inflammation by promoting tissue resident memory T cells differentiation through EZH2
Journal of Translational Medicine (2024)
-
Nanotechnology of inhalable vaccines for enhancing mucosal immunity
Drug Delivery and Translational Research (2024)
-
High proportion of circulating CD8 + CD28- senescent T cells is an independent predictor of distant metastasis in nasopharyngeal carcinoma after radiotherapy
Journal of Translational Medicine (2023)
-
Tumor-targeted superantigens produce curative tumor immunity with induction of memory and demonstrated antigen spreading
Journal of Translational Medicine (2023)