Key Points
-
Under normal circumstances, formation of superoxide anions is kept under tight control by superoxide dismutase enzymes. These include the manganese (Mn) enzyme in mitochondria and the copper (Cu)/zinc (Zn) enzyme that is present in the cytosol or on extracellular surfaces.
-
Superoxide anions are formed by means of several pathways, including through normal cellular respiration, by inflammatory cells, by endothelial cells and in the metabolism of arachidonic acid.
-
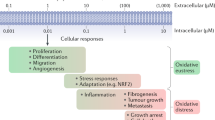
Extensive scientific research over the past twenty years has shown that, in acute and chronic inflammation, superoxide anions are produced at a rate that overwhelms the capacity of the endogenous superoxide dismutase enzyme-defence system to remove them. Such an imbalance results in superoxide-mediated damage.
-
Protective and beneficial roles of superoxide dismutase have been shown in a broad range of diseases, both preclinically and clinically. The results from the latter studies prove the concept that superoxide anions have an important role in human disease, and that their removal by the native enzyme does in fact result in beneficial outcomes.
-
Although the native enzymes have shown promising anti-inflammatory properties in both preclinical and clinical studies in various diseases, there were drawbacks and issues that were associated with the use of the native enzymes as therapeutic agents and as pharmacological tools.
-
On the basis that removing superoxide anions modulates the course of inflammation, synthetic, low-molecular-mass mimetics of the superoxide dismutase enzymes, which can overcome some of the limitations that are associated with the use of the native enzymes, have been developed as potential therapeutic agents.
Abstract
The list of pathophysiological conditions that are associated with the overproduction of superoxide anions expands every day. The most exciting realization is that there seems to be a similarity between the tissue injury that is observed in various disease states, as superoxide anions produce tissue injury and associated inflammation in all tissues in similar ways. Tissue injury and inflammation form the basis of many disease pathologies, including ischaemia and reperfusion injuries, radiation injury, hyperoxic lung damage and atherosclerosis. This commonality provides a unique opportunity to manipulate numerous disease states with an agent that removes superoxide anions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huber, W. et al. Some chemical and pharmacological properties of a novel anti-inflammatory protein. Toxicol. Appl. Pharmacol. 12, 308–324 (1968).This paper describes the pharmacological recognition that Orgotein has potent anti-inflammatory activity
McCord, J. M. & Fridovich, I. Superoxide dismutase: an enzymatic function for erythrocuprein. J. Biol. Chem. 244, 6049–6055 (1969).The recognition that the corresponding protein to Orgotein catalyses the dismutation of superoxide anions into hydrogen peroxide and molecular oxygen, which indicates that removal of superoxide is beneficial in inflammation.
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 13, 43–47 (1996).
Carlsson, L. M., Jonsson, J., Edlundand, T. & Marklund, S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl Acad. Sci. USA 92, 6264–6268 (1995).References 3 and 4 show that the Cu/Zn SOD is not necessary for normal cell survival, but is required under physiologically stressful conditions after injury.
Lebovitz, R. M. et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide-deficient mice. Proc. Natl Acad. Sci. USA 93, 9782–9787 (1996).
Melov, S. et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl Acad. Sci. USA 96, 846–851 (1999).References 5 and 6 highlight the importance of the mitocondrial Mn SOD enzyme for cell survival — knockout animals are not viable.
Droy-Lefaix, M. T., Drouet, Y., Geraud, G., Hosfod, D. & Braquet, P. Superoxide dimutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia–reperfusion. Free Radic. Res. Commun. 12-13, 725–735 (1991).
Haglind, E., Xia, G. & Rylander, R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock 42, 83–91 (1994).
Fantone, J. C. & Ward, P. A. A review: role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 107, 395–418 (1982).
Deitch, E. A., Bridges, W., Berg, R., Specian, R. D. & Granger, N. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J. Trauma 30, 942–951 (1990).
Boughton-Smith, N. K., Evans, S. M., Laszlo, F., Whittle, B. J. & Moncada, S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 110, 1189–1195 (1993).
Salvemini, D. et al. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J Pharmacol. 303, 217–220 (1996).
Salvemini, D. et al. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 127, 685–692 (1999).
Dix, T. A. et al. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry 35, 4578–4583 (1996).
Beckman, J. S., Beckman, T. W., Chen, J., Marshalland, P. A. & Freeman, B. A. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl Acad. Sci. USA 87, 1620–1624 (1990).
Ischiropoulos, H., Zhu, L. & Beckman, J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 298, 446–451 (1992).
Crow, J. P. & Beckman, J. S. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv. Pharmacol. 34, 17–43 (1995).
Salvemini, D., Wang, Z.-Q., Stern, M. K., Currie, M. G. & Misko, T. P. Peroxynitrite decomposition catalysts: novel therapeutics for peroxynitrite-mediated pathology. Proc. Natl Acad. Sci. USA 95, 2659–2663 (1998).
Macarthur, H., Westfall, T. C., Riley, D. P., Misko, T. P. & Salvemini, D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc. Natl Acad. Sci. USA 97, 9753–9758 (2000).
Klug-Roth, D., Fridovich, I. & Rabani, J. Pulse radiolytic investigations of superoxide catalyzed disproportionation. Mechanism for bovine superoxide dismutase. J. Am. Chem. Soc. 95, 2786–2790 (1973).
Waldo, G. S. & Penner-Hahn, J. E. Mechanism of manganese catalase peroxide disproportionation: determination of manganese oxidation states during turnover. Biochemistry 7, 1507–1512 (1995).
Krall, J., Bagley, A. C., Mullenbach, G. T., Hallewell, R. A. & Lynch, R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J. Biol. Chem. 263, 1910–1914 (1988).
Ho, Y. S., Vincent, R., Dey, M. S., Slot, J. W. & Crapo, J. D. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am. J. Respir. Cell Mol. Biol. 18, 538–547 (1998).
Sohal, R. S., Agarwal, A., Agarwal, S. & Orr, W. C. Simultaneous overexpression of copper- and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 270, 15671–15674 (1995).
Halliwell, B. & Gutteridge, J. M. C. in Free Radicals in Biology and Medicine (eds Baum, H., Gergely, J. & Fanburg, B. L.) 89–193 (Oxford Univ. Press, Oxford, 1985).
Maxwell, S. R. J. Prospects for the use of antioxidant therapies. Drugs 49, 345–361 (1995).
McCord, J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science 185, 529–531 (1974).
Werns, S. W. et al. Sustained limitation by superoxide dismutase of canine myocardial injury due to ischemia followed by reperfusion. J. Cardiovasc. Pharmacol. 11, 36–44 (1988).
Omar, B. A. & McCord, J. M. Interstitial equilibration of superoxide dismutase correlates with its protective effect in the isolated rabbit heart. J. Mol. Cell. Cardiol. 23, 149–159 (1991).
McCord, J. M. Superoxide dismutase: rationale for use in reperfusion injury and inflammation. J. Free Radic. Biol. Med. 2, 307–310 (1986).
Ando, Y., Inoue, M., Hirota, M., Morino, Y. & Araki, S. Effect of superoxide dismutase derivative on cold-induced brain edema. Brain Res. 477, 286–291 (1989).
Chan, P. H., Yang, G. Y., Chen, S. F., Carlson, E. & Epstein, C. J. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing superoxide dismutase. Ann. Neurol. 29, 482–486 (1991).
Yang, G. et al. Human copper–zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25, 165–170 (1994).
Zweier, J. L. Prevention of reperfusion-induced, free radical-mediated acute endothelial injury by superoxide dismutase as an effective tool to delay/prevent chronic renal allograft failure: a review. Transplant. Proc. 29, 2567–2568 (1997).
Oyanagui, Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem. Pharmacol. 25, 1465–1472 (1976).
Droy-Lefaix, M. T., Drouet, Y., Geraud, G., Hosford, D. & Braquet, P. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia–reperfusion. Free Radic. Res. Commun. 12–13, 725–735 (1991).
Shingu, M. et al. Anti-inflammatory effects of recombinant human manganese superoxide dismutase on adjuvant arthritis in rats. Rheumatol. Int. 14, 77–81 (1994).
Bravard, A. et al. SOD2: a new type of tumor-suppressor gene? Int. J. Cancer 51, 476–480 (1992).
Church, S. L. et al. Increased manganese superoxide dismutase expression suppresses the malignant human melanoma cells. Proc. Natl Acad. Sci. USA 90, 3113–3117 (1993).
St Clair, D. K., Oberley, T. D., Muse, K. E. & St Clair, W. H. Expression of manganese superoxide dismutase promotes cellular differentiation. Free Radic. Biol. Med. 16, 275–282 (1994).
Safford, S. E., Oberley, T. D., Urano, M. & St Clair, D. K. Suppression of fibrosarcoma metastasis by elevated expression of manganese dismutase. Cancer Res. 54, 4261–4265 (1994).
Yoshizaki, N. et al. Suppressive effect of recombinant human Cu,Zn-superoxide dismutase on lung metastasis of murine tumor cells. Int. J. Cancer 57, 287–292 (1994).
Flores, S. C. et al. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl Acad. Sci. USA 90, 7632–7636 (1993).
Edas, M. A. et al. Clastogenic factors in plasma of HIV-1 infected patients activate HIV-1 replication in vitro: inhibition by superoxide dismutase. Free Radic. Biol. Med. 23, 571–578 (1997).
Mollace, V. et al. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 24, 411–416 (2001).
Salminen, U. et al. Superoxide dismutase in development of obliterative bronchiolitis. Transplant. Proc. 33, 2477 (2001).
Barnes, J. P. Chronic obstructive pulmonary disease. N. Engl. J. Med. 343, 269–280 (2000).
Nishiguchi, K. et al. Pharmaceutical studies for gene therapy: expression of human Cu,Zn-superoxide dismutase gene transfected by lipofection in rat skin fibroblasts. Biol. Pharm. Bull. 19, 1073–1077 (1996).
Batinic-Haberle, I., Benov, L., Spasojevic, I. & Fridovich, I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 273, 24521–24528 (1998).
Lawrence, G. D. & Sawyer, D. T. Potentiometric titrations and oxidation–reduction potentials of manganese and copper–zinc superoxide dismutases. Biochemistry 18, 3045–3050 (1979).
Day, B. J., Shawen, S., Liochev, S. I. & Crapo, J. D. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther. 275, 1227–1232 (1995).
Day, B. J., Fridovich, I. & Crapo, J. D. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 347, 256–262 (1997).
Day, B. J., Batinic-Haberle, J. & Crapo, J. D. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 26, 730–736 (1999).
Faulkner, K. M., Liochev, S. I. & Fridovich, I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 269, 23471–23476 (1994).References 54 and 59 show that stable compounds with SOD activity can function in vivo as SODs and ameliorate inflammation in in vivo animal models of inflammation. That these same Mn iii porphyrin-based catalysts are not selective for superoxide anions, but also react with hydrogen peroxide, is pointed out in reference 52. So, the use of Mn iii porphyrin-based SOD mimetics limits the ability to determine whether superoxide or hydrogen peroxide are the pro-inflammatory mediators.
Szabó, C., Day, B. J. & Salzman, A. L. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages, using a novel mesoporphyrin superoxide dismutase analog and peroxynitrite scavenger. FEBS Lett. 381, 82–86 (1996).
Dolphin, D., Forman, A., Borg, D. C., Fajer, J. & Felton, R. H. Compounds I of catalase and horse radish peroxidase: π-cation radicals. Proc. Natl Acad. Sci. USA 68, 614–618 (1971).
Gardner, P. R., Nguyen, D. D. & White, C. W. Superoxide scavenging by Mn(II/III) tetrakis (1-methyl-4-pyridyl) porphyrin in mammalian cells. Arch. Biochem. Biophys. 325, 20–28 (1996).
Misko, T. P. et al. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 273, 15646–15653 (1998).
Cuzzocrea, S., Zingarelli, B., Costantino, G. & Caputi, A. P. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-pleurisy. Free Radic. Biol. Med. 26, 25–33 (1999).
Zingarelli, B., Day, B. J., Crapo, J. D., Salzman, A. L. & Szabo, C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol. 120, 259–267 (1997).
Szabo, C. Potential role of the peroxynitrate– poly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock 9, 341–344 (1998).
Baudry, M. et al. Salen–manganese complexes are superoxide dismutase-mimics. Biochem. Biophys. Res. Commun. 192, 964–968 (1993).
Doctrow, S. R. et al. Salen–manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv. Pharmacol. 38, 247–269 (1997).
Malfroy, B. et al. Prevention and suppression of autoimmune encephalomyelitis by EUK-8, a synthetic catalytic scavenger of oxygen-reactive metabolites. Cell. Immunol. 177, 62–68 (1997).
Baker, K. et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 284, 215–221 (1998).
Bianca, R. et al. Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med. Sci. Monit. 8, BR1–BR7 (2002).
Henke, S. L. Superoxide dismutase mimics as future therapeutics. Exp. Opin. Ther. Patents 9, 169 (1999).
Riley, D. P., Henke, S. L., Lennon, P. J. & Aston, K. Computer-aided design (CAD) of synzymes: use of molecular mechanics (MM) for the rational design of superoxide dismutase mimics. Inorg. Chem. 38, 1908–1917 (1999).
Riley, D. P. Rational design of synthetic enzymes and their potential utility as human pharmaceuticals: development of Mn(II)-based superoxide dismutase mimics. Adv. Supramol. Chem. 6, 217–244 (1999).
Salvemini, D. et al. A non-peptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science 286, 304–306 (1999).A key reference that shows, for the first time, that a selective catalyst for the dismutation of superoxide anions has in vivo efficacy in animal models of inflammation. This shows that selective removal of superoxide anions, not the removal of hydrogen peroxide or hypochlorite, is the event in blocking inflammation, and that superoxide is a key initiator of reactive oxygen species and mediator of inflammation.
Salvemini, D. et al. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br. J. Pharmacol. 132, 815–827 (2001).
Riley, D. P. et al. Synthesis, characterization and stability of manganese (II) C-substituted 1,4,7,10,13-pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 35, 5213–5231 (1996).
Riley, D. P., Lennon, P. J., Neumann, W. L. & Weiss, R. H. Toward the rational design of superoxide dismutase mimics: mechanistic studies for the elucidation of substituent effects on the catalysis activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 119, 6522–6528 (1997).
Cuzzocrea, S. et al. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 132, 19–29 (2001).
Salvemini, D. et al. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis Rheum. 44, 2909–2921 (2001).
Squadrito, G. L. & Pryor, W. A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 96, 203–206 (1995).
Salvemini, D., Jensen, M. P., Riley, D. P. & Misko, T. P. Therapeutic manipulations of peroxynitrite. Drug News Persp. 11, 204–214 (1988).
Stern, M. K., Jensen, M. P. & Kramer, K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 118, 8735–8736 (1996).
Beckman, J. S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 9, 836–844 (1996).
Ischiropoulos, H., Al-Mehdi, A. B. & Fisher, A. B. Reactive species in ischemic rat lung injury: contribution of peroxynitrite. Am. J. Physiol. 269, 158–164 (1995).
Salvemini, D., Jensen, M. P., Riley, D. P. & Misko, T. P. Therapeutic manipulation of peroxinitrite. Drug News Persp. 11, 204–214 (1998).
Gryglewski, R. J., Palmer, R. M. & Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320, 454–456 (1986).Reference 82 is the seminal discovery that superoxide anions interact with, and destroy, the biological activity of the endothelium-derived relaxing factor (now known as nitric oxide), while generating the potent cytotoxic mediator peroxynitrite, as indicated in reference 15 . These references indicate that removal of superoxide anions preserves the beneficial activity of nitric oxide, while inhibiting the formation of cytotoxic peroxynitrite.
Salvemini, D. et al. NO activates cyclooxygenase enzymes. Proc. Natl Acad. Sci. USA 90, 7240–7244 (1993).
Salvemini, D., Marino, M. H. & Seibert, K. Activation of the cyclooxygenase pathway by nitric oxide: new concepts of inflammation and therapy. Drug News Persp., 204–219 (1996).
Inoue, S. & Kawanishi, S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 371, 86–88 (1995).
Salgo, M. G., Bermudez, E., Squadrito, G. & Pryor, W. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Arch. Biochem. Biophys. 322, 500–505 (1995).
Szabó, C. & Dawson, V. L. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia–reperfusion. Trends Pharmacol. Sci. 19, 287–298 (1999).
Cuzzocrea, S. et al. Protective effects of 3-aminobenzamide, an inhibitor of poly(ADP-ribose) synthase in carrageenan-induced models of local inflammation. Eur. J. Pharmacol. 342, 67–76 (1998).
Szabó, C. et al. Inhibition of poly(ADP-ribose) synthetase exerts anti-inflammatory effects and inhibits neutrophil recruitment. J. Exp. Med. 186, 1041–1049 (1997).
Warren, J. S., Yabroff, K. R., Mandel, D. M., Johnson, K. J. & Ward, P. A. Role Of O2− in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic. Biol. Med. 8, 163–172 (1990).
Al-Shabanah, O. A., Mansour, M. A. & Elmazar, M. M. Enhanced generation of leukotriene B4 and superoxide radical from calcium ionophore (A23187) stimulated human neutrophils after priming with interferon-α. Res. Commun. Mol. Pathol. Pharmacol. 106, 115–128 (1999).
Lowe, D., Pagel, P. S., McGough, M. F., Hettrick, D. A. & Warltier, D. C. Comparison of the cardiovascular effects of two novel superoxide dismutase mimetics, SC-55858 and SC-54417, in conscious dogs. Eur. J. Pharmacol. 304, 81–86 (1996).
Cuzzocrea, S., Riley, D. P., Caputi, A. P. & Salvemini, D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 53, 135–159 (2001).
Cuzzocrea, S. et al. Beneficial effects of peroxynitrite decomposition catalyst in rat model of splanchnic artery occlusion and reperfusion. FASEB J. 14, 1061–1072 (2000).
Volk, T., Gerst, J., Faust-Belbe, G., Stroehmann, A. & Kox, W. J. Monocyte stimulation by reactive oxygen species: role of superoxide and intracellular Ca2+. Inflamm. Res. 48, 544–549 (1999).
Haddad, J. J. & Land, S. C. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-α biosynthesis. Br. J. Pharmacol. 135, 520–536 (2002).
McInnis, J. et al. The role of superoxide and NF-κB signaling in N-methyl-d-aspartate-induced necrosis and apoptosis. J. Pharmacol. Exp. Ther. 301, 1–10 (2002).
Baeuerle, P. A. & Henkel, T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12, 141–179 (1994).
Barnes, P. J. & Karin, M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J Med. 336, 1066–1071 (1997).
Niwa, Y., Somiya, K., Michelson, A. M. & Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic. Res. Commun. 1, 137–153 (1985).
Flohe, L. Superoxide dismutase for therapeutic use: clinical experience, dead ends and hopes. Mol. Cell. Biochem. 84, 123–131 (1988).
Goebel, K. M., Storck, U. & Neurath, F. Intrasynovial Orgotein therapy in rheumatoid arthritis. Lancet 1, 1015–1017 (1981).
Goebel, K. M. & Storck, U. Effect of intra-articular Orgotein versus a corticosteroid on rheumatoid arthritis of the knees. Am. J. Med. 74, 124–128 (1983).
Lund-Olesen, K. & Menander-Huber, K. B. Intra-articular Orgotein therapy in osteoarthritis of the knee. A double-blind, placebo-controlled trial. Drug Res. 8, 1199–1203 (1983).
Gammer, W. & Broback, L. G. Clinical comparison of Orgotein and methylprednisolone acetate in the treatment of osteoarthrosis of the knee joint. Scand. J. Rheumatol. 13, 108–112 (1984).
McIlwain, H. et al. Intra-articular Orgotein in osteoarthritis of the knee: a placebo-controlled efficacy, safety, and dosage comparison. Am. J. Med. 87, 295–300 (1989).
Mazieres, B., Masquelier, A. M. & Capron, M. H. A French controlled multicenter study of intraarticular Orgotein versus intraarticular corticosteroids in the treatment of knee osteoarthritis: a one-year followup. J. Rheumatol. 18, 134–137 (1991).
Lin, Y., Pape, H. D. & Friedrich, R. Use of superoxide dismutase (SOD) in patients with temporomandibular joint dysfunction — a preliminary study. J. Oral Maxillofac. Surg. 23, 428–429 (1994).References 102–108 show that removal of superoxide by Orgotein is anti-inflammatory.
Pascu, O. & Dejica, D. Oxygen free radicals and duodenal ulcer pain. Preliminary data. Med. Interne 25, 81–84 (1987).
Edsmyr, F., Huber, W. & Menander, K. B. Orgotein efficacy in ameliorating side effects due to radiation therapy. I. Double-blind, placebo-controlled trial in patients with bladder tumors. Curr. Ther. Res. Clin. Exp. 19, 198–211 (1976).
Marberger, H., Huber, W., Bartsch, G., Schulte, T. & Swoboda, P. Orgotein: a new antiinflammatory metalloprotein drug evaluation of clinical efficacy and safety in inflammatory conditions of the urinary tract. Int. Urol. Nephrol. 6, 61–74 (1974).
Housset, M., Faillet, F., Michelson, A. M. & Puget, K. Action of liposomal superoxide dismutase on measurable radiation-induced fibrosis. Ann. Med. Interne (Paris) 140, 365–367 (1989).
Villasor, R. P. in The Pathology of Oxygen (ed. Autor, A. P.) 303–314 (Academic, New York, 1982).
Perderau, B. et al. Superoxide dismutase (Cu/Zn) in cutaneous application in the treatment of radiation-induced fibrosis. Bull. Cancer 81, 659–669 (1994).
Delanian, S. et al. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother. Oncol. 32, 12–20 (1994).
Sanchiz, F. et al. Prevention of radioinduced cystitis by Orgotein: a randomized study. Anticancer Res. 16, 2025–2028 (1996).
Marberger, H., Bartsch, G., Huber, W., Menander, K. B. & Schulte, T. Orgotein: a new drug for the treatment of radiation cystitis. Curr. Ther. Res. Clin. Exp. 18, 466–475 (1975).
Marberger, H., Huber, W., Menander-Huber, K. B. & Bartsch, G. Orgotein, a new drug for the treatment of Peyronie's disease. Eur. J. Rheumatol. Inflamm. 4, 244–249 (1981).
Menander-Huber, K. B., Edsmyr, F. & Huber, W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Urol. Res. 6, 255–257 (1978).
Babior, B. M. Superoxide: a two-edged sword. Braz. J. Med. Biol. Res. 30, 141–155 (1982).References 112–120 show that removal of superoxide by Orgotein is protective against radiation-induced damage, and is effective in reversing established fibrosis post-radiation therapy in human clinical trials. Along with references 102–108 , these papers support the concept that superoxide has an important role in human disease.
Riley, D. P., Rivers, W. J. & Weiss, R. H. Stopped-flow kinetic analysis for monitoring superoxide decay in aqueous systems. Anal. Biochem. 196, 344–349 (1991).
Evans, S. M. & Whittle, B. J. Interactive roles of superoxide and inducible nitric oxide synthase in rat intestinal injury provoked by non-steroidal anti-inflammatory drugs. Eur. J. Pharmacol. 429, 287–296 (2001).
Lefaix, J. L. et al. Successful treatment of radiation induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int. J. Radiat. Oncol. Biol. Phys. 35, 305–312 (1996)
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
OMIM
LINKS
Glossary
- ENZYME MIMETIC
-
A chemical entity that exactly copies the functional property of an enzyme.
- DISMUTATION
-
A chemical reaction in which two molecules of the same compound react together to produce two new molecules.
- NEUTROPHILS
-
Circulating white blood cells in the granulocyte series that represent from 55–65% of the total number of leukocytes.
- HYPOTENSION
-
Subnormal arterial blood pressure.
- REDUCING EQUIVALENT
-
A reducing agent that provides a source of electrons.
- METALLOPORPHYRIN
-
A metal complex with a porphyrin ligand — a completely unsaturated macrocyclic tetrapyrrole ligand that contains a π-conjugated ring system of the class that includes the iron-containing oxygen-binding site (haem) of haemoglobin.
- FREE RADICAL
-
Any species capable of independent existence that contains one or more unpaired electrons — an unpaired electron being one that is alone in an orbital.
- CATALASE
-
A haem-containing protein (enzyme) that catalytically converts hydrogen peroxide to water and dioxygen.
- CARRAGEENAN
-
The name given to a family of sulphated polysaccharides that are obtained from various seaweeds.
- REACTIVE OXYGEN SPECIES
-
These include O2●−, OH and H2O2, as well as unstable intermediates that are produced during the peroxidation of lipids.
- CYCLIC VOLTAMETRY EXPERIMENT
-
A standard electrochemical technique for measuring oxidation.
- INTRA-ARTICULAR INJECTION
-
An injection into the articular space.
- ELECTROPARAMAGNETIC RESONANCE
-
(EPR). A name applied to a routine technique that is used to study molecules and ions that contain unpaired electrons by observing the magnetic fields at which they are in resonance with monochromatic radiation.
Rights and permissions
About this article
Cite this article
Salvemini, D., Riley, D. & Cuzzocrea, S. Sod mimetics are coming of age. Nat Rev Drug Discov 1, 367–374 (2002). https://doi.org/10.1038/nrd796
Issue Date:
DOI: https://doi.org/10.1038/nrd796
This article is cited by
-
Allicin and Omega-3 fatty acids attenuates acetaminophen mediated renal toxicity and modulates oxidative stress, and cell apoptosis in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment
Nature Communications (2022)
-
The effects of apigenin administration on the inhibition of inflammatory responses and oxidative stress in the lung injury models: a systematic review and meta-analysis of preclinical evidence
Inflammopharmacology (2022)
-
Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats
Environmental Science and Pollution Research (2020)
-
A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development
Nature Communications (2019)