Key Points
-
The adaptation of mammalian cells to low oxygen conditions is mediated in large part by the transcriptional induction of gene expression. Hypoxia-inducible factor (HIF) is crucial in the transcriptional response of cells to hypoxia.
-
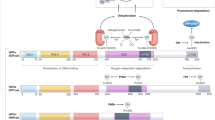
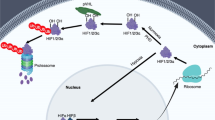
The HIF transcription factor is composed of an oxygen-labile α-subunit and a constitutively expressed β-subunit. HIF belongs to a family of heleix–loop–helix PER/SIM/ARNT (HLH-PAS) transcription factors.
-
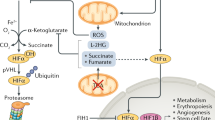
HIF-1α stability and activity are regulated by post-translational modifications, chaperone function and alternative splicing. These pathways have generated new targets for high-throughput screening strategies.
-
In transformed cells, HIF is regulated by oncogenic and tumour-suppressor gene mutations that cause it to become stabilized under aerobic conditions.
-
HIF-1 is involved in the inflammatory response. Inhibition of HIF-1 in myeloid cells can ameliorate and even prevent inflammation, indicating a new role for HIF modulators.
-
Development of HIF-inducing compounds provides a new approach to the regulation of erythropoietin and erythropoiesis in cancer patients, of angiogenesis in cardiovascular disease and of ischaemic injury in renal patients. Small molecules that affect prolyl hydroxylation or ubiquitylation provide a prime target for such regulation.
Abstract
Sensing and responding to fluxes in oxygen tension is perhaps the single most important variable in physiology, and animal tissues have developed a number of essential mechanisms to cope with the stress of low physiological oxygen levels, or hypoxia. Among these coping mechanisms is the response mediated by the hypoxia-inducible transcription factor, or HIF-1. HIF-1 is an essential component in changing the transcriptional repertoire of tissues as oxygen levels drop, and could prove to be a very important target for drug development, as treatments evolve for diseases, such as cancer, heart disease and stroke, in which hypoxia is a central aspect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Semenza, G. L., Roth, P. H., Fang, H. -M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23767 (1994).
Goldberg, M. A., Glass, G. A., Cunningham, J. M. & Bunn, H. F. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc. Natl Acad. Sci. USA 84, 7972–7976 (1987).
Goldberg, M. A., Dunning, S. P. & Bunn, H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242, 1412–1415 (1988).
Shweiki, D., Itin, A., Soffer, D. & Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843–845 (1992).
Graham, C. H., Forsdike, J., Fitzgerald, C. J. & Macdonald-Goodfellow, S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int. J. Cancer 80, 617–623 (1999).
Bodi, I., Bishopric, N. H., Discher, D. J., Wu, X. & Webster, K. A. Cell-specificity and signaling pathway of endothelin-1 gene regulation by hypoxia. Cardiovas. Res. 30, 975–984 (1995).
Semenza, G. L., Koury, S. T., Nejfelt, M. K., Gearhart, J. D. & Antonarakis, S. E. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc. Natl Acad. Sci. USA 88, 8725–8729 (1991).
Imagawa, S., Goldberg, M. A., Doweiko, J. & Bunn, H. F. Regulatory elements of the erythropoietin gene. Blood 77, 278–285 (1991).
Wang, G. L. & Semenza, G. L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 (1995). First biochemical purification of HIF-1, identified two subunits.
Jiang, B. H., Rue, E., Wang, G. L., Roe, R. & Semenza, G. L. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271, 17771–17778 (1996).
Wenger, R. H., Kvietikova, I., Rolfs, A., Gassmann, M. & Marti, H. H. Hypoxia-inducible factor-1α is regulated at the post-mRNA level. Kidney Int. 51, 560–563 (1997).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998).
Pugh, C. W., O'Rourke, J. F., Nagao, M., Gleadle, J. M. & Ratcliffe, P. J. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the α subunit. J. Biol. Chem. 272, 11205–11214 (1997).
Srinivas, V., Zhang, L. P., Zhu, X. H. & Caro, J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor α (HIF-α) proteins. Biochem. Biophys. Res. Commun. 260, 557–561 (1999).
Jiang, B. H., Zheng, J. Z., Leung, S. W., Roe, R. & Semenza, G. L. Transactivation and inhibitory domains of hypoxia-inducible factor 1α. Modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 272, 19253–19260 (1997).
Ema, M., Taya, S., Yokotani, N., Sogawa, K., Matsuda, Y. & Fujii-Kuriyama, Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl Acad. Sci. USA 94, 4273–4278 (1997).
Flamme, I., Frolich, T. & Risau, W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J. Cell. Physiol. 173, 206–210 (1997).
Hogenesch, J. B. et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J. Biol. Chem. 272, 8581–8593 (1997).
Tian, H., McKnight, S. L. & Russell, D. W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11, 72–82 (1997).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 7, 205–213 (1998).
Salceda, S. & Caro, J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272, 22642–22647 (1997).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). This paper made the important finding that the VHL tumour-suppressor gene targets HIF-1α subunit for ubiquitin-mediated degradation under poxic conditions.
Ohh, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nature Cell Biol. 2, 423–427 (2000).
Gassmann, M., Chilov, D. & Wenger, R. H. Regulation of the hypoxia-inducible factor-1α. ARNT is not necessary for hypoxic induction of HIF-1α in the nucleus. Adv. Exp. Med. Biol. 475, 87–99 (2000).
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657–661 (1999).
Kibel, A., Iliopoulos, O., DeCaprio, J. A. & Kaelin, W. G. Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 269, 1444–1446 (1995).
Lonergan, K. M. et al. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell. Biol. 18, 732–741 (1998).
Pause, A. et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl Acad. Sci. USA 94, 2156–2161 (1997).
Pause, A., Peterson, B., Schaffar, G., Stearman, R. & Klausner, R. D. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc. Natl Acad. Sci. USA 96, 9533–9538 (1999). Provides insight into how VHL targets HIF-1α for ubiquitin-mediated degradation.
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999).
Koepp, D. M., Harper, J. W. & Elledge, S. J. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97, 431–434 (1999).
Tyers, M. & Jorgensen, P. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr. Opin. Genet. Dev. 10, 54–64 (2000).
Duan, D. R. et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269, 1402–1406 (1995).
Tanimoto, K., Makino, Y., Pereira, T. & Poellinger, L. Mechanism of regulation of the HIF-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19, 4298–4309 (2000).
Cockman, M. E. et al. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275, 25733–25741 (2000).
Kamura, T. et al. Activation of HIF1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc. Natl Acad. Sci. USA 97, 10430–10435 (2000).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Ivan, M. et al. HIF-α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001). One of two important papers that initially demonstrated that the oxygen sensor for HIF-1 degradation is hydroxylation of a crucial proline residue.
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001). The other important paper showing that proline hydroxylation regulates the oxygen lability of HIF-1α.
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Ema, M. et al. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 18, 1905–1914 (1999).
Gu, J., Milligan, J. & Huang, L. E. Molecular mechanism of hypoxia-inducible factor 1α–p300 interaction. A leucine-rich interface regulated by a single cysteine. J. Biol. Chem. 276, 3550–3554 (2001).
Kung, A. L., Wang, S., Klco, J. M., Kaelin, W. G. & Livingston, D. M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nature Med. 6, 1335–1340 (2000).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295, 858–861 (2002). Indicates that hydroxylation of different residues can regulate HIF-1 transactivation as well as stability.
McNeill, L. A. et al. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the β-carbon of asparagine-803. Biochem J. 367, 571–575 (2002).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Hewitson, K. S. et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 277, 26351–26355 (2002).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001).
Huang, L. E., Arany, Z., Livingston, D. M. & Bunn, H. F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. J. Biol. Chem. 271, 32253–32259 (1996).
Carrero, P. et al. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1α. Mol. Cell. Biol. 20, 402–415 (2000).
Lando, D., Pongratz, I., Poellinger, L. & Whitelaw, M. L. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1α and the HIF-like factor. J. Biol. Chem. 275, 4618–4627 (2000).
Jeong, J. W. et al. Regulation and destabilization of HIF-1α by ARD1-mediated acetylation. Cell 111, 709–720 (2002).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J. Biol. Chem. 277, 32405–32408 (2002).
Chun, Y. S. et al. A new HIF-1α variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J. Cell Sci. 114, 4051–4061 (2001).
Chun, Y. S., Choi, E., Kim, T. Y., Kim, M. S. & Park, J. W. A dominant-negative isoform lacking exons 11 and 12 of the human hypoxia-inducible factor-1α gene. Biochem J. 362, 71–79 (2002).
Tanguay, R. L., Andreasen, E., Heideman, W. & Peterson, R. E. Identification and expression of alternatively spliced aryl hydrocarbon nuclear translocator 2 (ARNT2) cDNAs from zebrafish with distinct functions. Biochim. Biophys. Acta 1494, 117–128 (2000).
Semenza, G. L. Involvement of hypoxia-inducible factor 1 in human cancer. Intern. Med. 41, 79–83 (2002).
Karni, R., Dor, Y., Keshet, E., Meyuhas, O. & Levitzki, A. Activated pp60c-Src leads to elevated HIF-1α expression under normoxia. J. Biol. Chem. 277, 42919–42925 (2002).
Lu, H., Forbes, R. A. & Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 277, 23111–23115 (2002).
Semenza, G. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 64, 993 (2002).
Laughner, E., Taghavi, P., Chiles, K., Mahon, P. C. & Semenza, G. L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21, 3995–4004 (2001).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002). A thorough review on the roles of hypoxia in malignant progression and as a target for cancer therapy.
Ryan, H. E., Lo, J. & Johnson, R. S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3015 (1998). First demonstration that genetic deletion of HIF-1 can retard tumour growth.
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A. & Simon, M. C. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 (1997). First demonstration of the importance of HIF-1 in controlling angiogenesis and metabolism.
Sun, X. et al. Gene transfer of antisense hypoxia inducible factor-1α enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 8, 638–645 (2001).
Isaacs, J. S. et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J. Biol. Chem. 277, 29936–29944 (2002).
Mabjeesh, N. J. et al. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 62, 2478–2482 (2002).
Blancher, C., Moore, J. W., Robertson, N. & Harris, A. L. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1α, HIF-2α, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3'-kinase/Akt signaling pathway. Cancer Res. 61, 7349–7355 (2001).
Mazure, N. M., Chen, E. Y., Yeh, P., Laderoute, K. R. & Giaccia, A. J. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 56, 3436–3440 (1996).
Kurebayashi, J. et al. A radicicol derivative, KF58333, inhibits expression of hypoxia-inducible factor-1α and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Jpn J. Cancer Res. 92, 1342–1351 (2001).
Neckers, L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 8, S55–S61 (2002).
Welsh, S. et al. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1α and vascular endothelial growth factor formation. Mol. Cancer Ther. 2, 235–243 (2003).
Jung, F. et al. Hypoxic induction of the hypoxia-inducible factor is mediated via the adaptor protein Shc in endothelial cells. Circ. Res. 91, 38–45 (2002).
Chan, D. A., Sutphin, P. D., Denko, N. C. & Giaccia, A. J. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1α. J. Biol. Chem. 277, 40112–40117 (2002).
Jiang, B. H., Agani, F., Passaniti, A. & Semenza, G. L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 57, 5328–5335 (1997).
Cohen-Jonathan, E. et al. The farnesyltransferase inhibitor L744,832 reduces hypoxia in tumors expressing activated H-ras. Cancer Res. 61, 2289–2293 (2001).
Maxwell, P. H., Pugh, C. W. & Ratcliffe, P. J. The pVHL-HIF-1 system. A key mediator of oxygen homeostasis. Adv. Exp. Med. Biol. 502, 365–376 (2001). A detailed review on VHL and HIF interaction.
Arbiser, J. L. et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl Acad. Sci. USA 94, 861–866 (1997).
Jiang, B. H. et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12, 363–369 (2001).
Mazure, N. M., Chen, E. Y., Laderoute, K. R. & Giaccia, A. J. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90, 3322–3331 (1997).
Zundel, W. et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 14, 391–396 (2000).
Cantley, L. C. & Neel, B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. USA 96, 4240–4245 (1999).
Alvarez-Tejado, M. et al. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J. Biol. Chem. 277, 13508–13517 (2002).
An, W. G. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392, 405–408 (1998).
Blagosklonny, M. V. et al. p53 inhibits hypoxia-inducible factor-stimulated transcription. J. Biol. Chem. 273, 11995–11998 (1998).
Thomas, G. & Hall, M. N. TOR signalling and control of cell growth. Curr. Opin. Cell Biol. 9, 782–787 (1997).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Berra, E., Pages, G. & Pouyssegur, J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 19, 139–145 (2000).
Sodhi, A., Montaner, S., Miyazaki, H. & Gutkind, J. S. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1α in rasV12 upregulation of VEGF. Biochem. Biophys. Res. Commun. 287, 292–300 (2001).
Feldser, D. et al. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 59, 3915–3918 (1999).
Zelzer, E. et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 17, 5085–5094 (1998).
Treins, C., Giorgetti-Peraldi, S., Murdaca, J., Semenza, G. L. & Van Obberghen, E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 277, 27975–27981 (2002).
Zhong, H. et al. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60, 1541–1545 (2000).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001).
Guba, M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Med. 8, 128–135 (2002).
Rapisarda, A. et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 62, 4316–4324 (2002). First published high-throughput screen for HIF-1 inhibitors.
Plowman, J. et al. Efficacy of the quinocarmycins KW2152 and DX-52-1 against human melanoma lines growing in culture kand in mice. Cancer Res. 55, 862–867 (1995).
Bunnell, C. A. et al. Phase I clinical trial of 7-cyanoquinocarcinol (DX-52-1) in adult patients with refractory solid malignancies. Cancer Chemother. Pharmacol. 48, 347–355 (2001).
Pribluda, V. S. et al. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 19, 173–179 (2000).
Mabjeesh, N. J. et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3, 363–375 (2003).
Jung, Y. J., Isaacs, J. S., Lee, S., Trepel, J. & Neckers, L. Microtubule disruption utilizes an NFκB-dependent pathway to stabilize HIF-1α protein. J. Biol. Chem. 278, 7445–7452 (2003).
Ko, F. N., Wu, C. C., Kuo, S. C., Lee, F. Y. & Teng, C. M. YC-1, a novel activator of platelet guanylate cyclase. Blood 84, 4226–4233 (1994).
Yeo, E. J. et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J. Natl Cancer Inst. 95, 516–525 (2003).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Folkman, J. & Cotran, R. Relation of vascular proliferation to tumor growth. Int. Rev. Exp. Pathol. 16, 207–248 (1976).
Brown, J. M. & Giaccia, A. J. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58, 1408–1416 (1998).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Vincent, K. A., Feron, O. & Kelly, R. A. Harnessing the response to tissue hypoxia: HIF-1α and therapeutic angiogenesis. Trends Cardiovasc. Med. 12, 362–367 (2002).
Vincent, K. A. et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 102, 2255–2261 (2000).
Belanger, A. J. et al. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor-γ angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1α. J. Mol. Cell. Cardiol. 34, 765–774 (2002).
Jiang, C. et al. Gene expression profiles in human cardiac cells subjected to hypoxia or expressing a hybrid form of HIF-1α. Physiol. Genomics 8, 23–32 (2002).
Nwogu, J. I. et al. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation 104, 2216–2221 (2001).
Park, S. et al. Hypoxia-induced gene expression occurs solely through the action of HIF-1α: The role of cytoplasmic trapping of HIF-2α. Mol. Cell. Biol. 23, 4959–4971 (2003).
Acknowledgements
We apologize for any references that have not been included that have contributed to our understanding of HIF. We would like to thank all the present and past members of our laboratories that contributed to the understanding of the role of hypoxia and HIF in normal tissue homeostasis and malignant progression. The work is supported by grants from the National Cancer Institute and the Auckland Cancer Research Society.
Author information
Authors and Affiliations
Corresponding author
Glossary
- HYPOXIA-RESPONSE ELEMENT
-
(HRE). Initially identified as a 50-base-pair sequence in the 3′ flanking region of the erythropoietin gene. Although the core DNA binding element is 5′-ACGTG-3′, flanking sequences are also important in HRE functionality. Approximately 30 genes have been found to possess HREs.
- HLH–PAS DOMAIN
-
HLH is a helix–loop–helix motif that facilitates dimerization and DNA binding and is found in a substantial number of transcription factors. The PAS domain was named after PER, ARNT and SIM proteins that represent the first proteins in which this motif was identified. Functionally, the PAS domain facilitates protein–protein interactions between family members.
- GAL4 DNA-BINDING PROTEIN
-
A transcriptional activator identified in yeast, which, because it is specific for yeast, is used to make fusion proteins to study mammalian transcriptional regulators.
- OXYGEN-DEPENDENT DEGRADATION
-
(ODD). The ODD domain of HIF-1α binds to VHL under aerobic conditions. Deletion of this domain results in a HIF-1α protein that is oxygen insensitive and constitutively expressed under aerobic conditions.
- UBIQUITIN-MEDIATED DEGRADATION
-
The energy-requiring process of covalently linking ubiquitin to lysine residues of a substrate protein to signal protein degradation.
- VON HIPPEL-LINDAU
-
(VHL). A tumour-suppressor gene that possesses two substrate-binding domains, -α and -β. The α-domain binds to elongin C and CUL2, proteins that possess sequence similarity with proteins known to be involved in ubiquitin-mediated degradation. The β-domain of VHL binds HIF-1α.
- PROLYL HYDROXYLATION
-
A protein modification mediated by an evolutionarily conserved group of iron-dependent enzymes termed prolyl hydroxylases (PhDs). As they require oxygen for their activity, they have been implicated as the oxygen sensor that regulates HIF-1α stabilization. Loss of PhD activity in Caenorhabditis elegans and, more recently, in mammalian cells, has resulted in stabilization of HIF-1α under aerobic conditions.
- ASPARAGINE HYDROXYLATION
-
This modification of HIF-1α on asparagine 803 has been implicated in the control of HIF-1 transactivation potential. The gene identified that controls this modification is termed FIH.
- ACETYLATION
-
Acetylation has previously been implicated in promoting transcriptional activation. By contrast, acetylation of HIF-1α on lysine 532 by ARD1 has been shown to be involved in its degradation by the proteasome.
- DEHYDROXYLASE
-
An enzyme that can remove the hydroxyl group from proline 564 and promote HIF-1α stabilization.
- DEUBIQUITINASE
-
An enzyme that promotes the removal of ubiquitin from a substrate protein such as HIF-1α through the cleavage of isopeptide bonds. The enzymatic activity of a HIF-1α deubiquitinase should increase HIF-1α stabilization.
- HYPOXIA-SPECIFIC CYTOTOXIN
-
A molecule whose cytotoxic activity is inhibited under aerobic conditions and increased under hypoxic conditions.
Rights and permissions
About this article
Cite this article
Giaccia, A., Siim, B. & Johnson, R. HIF-1 as a target for drug development. Nat Rev Drug Discov 2, 803–811 (2003). https://doi.org/10.1038/nrd1199
Issue Date:
DOI: https://doi.org/10.1038/nrd1199
This article is cited by
-
CDCA8 promotes bladder cancer survival by stabilizing HIF1α expression under hypoxia
Cell Death & Disease (2023)
-
Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
NSD1 promotes esophageal cancer tumorigenesis via HIF1α signaling
Cell Biology and Toxicology (2023)
-
Glycogen synthase kinase GSK3α promotes tumorigenesis by activating HIF1/VEGFA signaling pathway in NSCLC tumor
Cell Communication and Signaling (2022)
-
Hypoxia signaling in human health and diseases: implications and prospects for therapeutics
Signal Transduction and Targeted Therapy (2022)