Key Points
-
Pancreatic adenocarcinoma is a highly aggressive malignancy that shows profound resistance to extant treatments.
-
Genetic studies identified a signature molecular profile of this malignancy, consisting of mutations in KRAS, CDKN2A, TP53 and SMAD4/DPC4.
-
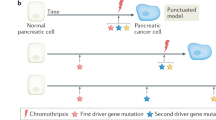
Pancreatic adenocarcinomas seem to arise from the progression of lesions that occur in the pancreatic ducts (pancreatic intraepithelial neoplasia, PanIN). Although the mutations listed above seem to occur in a temporal sequence in progressive PanIN stages, the specific biochemical and cellular events resulting from the mutations are not known.
-
This tumour type shows extensive genomic instability and aneuploidy. Telomere attrition and mutations in TP53 and BRCA2 are likely to contribute to these phenotypes.
-
There is ongoing study of the cell of origin in pancreatic adenocarcinoma. Although there is general agreement that the pancreatic ductal epithelial cell gives rise to this malignancy, there is evidence that transdifferentiation of other pancreatic cell types, such as acinar cells, might serve as an alternative route to pancreatic adenocarcinoma.
-
These tumours show an extensive proliferation of stromal fibroblasts and deposition of extracellular-matrix components (desmoplasia) that seem to promote growth and invasiveness. The molecular basis of this phenotype is not resolved, although TGF-β is thought to have a role.
-
Engineered mouse models have recapitulated some of the genetic and histological features of the human disease. The use of refined methodologies, such as tissue-specific mouse knockouts, should give insight into the biological and biochemical impact of tumour-suppressor gene loss or oncogene activation in pancreatic neoplasia.
Abstract
Pancreatic ductal adenocarcinoma is an aggressive and devastating disease, which is characterized by invasiveness, rapid progression and profound resistance to treatment. Advances in pathological classification and cancer genetics have improved our descriptive understanding of this disease; however, important aspects of pancreatic cancer biology remain poorly understood. What is the pathogenic role of specific gene mutations? What is the cell of origin? And how does the stroma contribute to tumorigenesis? A better understanding of pancreatic cancer biology should lead the way to more effective treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Niederhuber, J. E., Brennan, M. F. & Menck, H. R. The National Cancer Data Base report on pancreatic cancer. Cancer 76, 1671–1677 (1995).
Warshaw, A. L. & Fernandez-del Castillo, C. Pancreatic carcinoma. N. Engl. J. Med. 326, 455–465 (1992).
Ahrendt, S. A. & Pitt, H. A. Surgical management of pancreatic cancer. Oncology 16, 725–734; discussion 734, 736–738, 740, 743 (2002).
Kern, S. et al. A white paper: the product of a pancreas cancer think tank. Cancer Res. 61, 4923–4932 (2001).
Anderson, K. E., Potter, J. D. & Mack, T. M. in Cancer Epidemiology and Prevention (eds Schottenfeld, D. & Fraumeni, J. J.) 725–771 (Oxford University Press, New York, 1996).
Lynch, H. T. et al. Familial pancreatic cancer: a review. Semin. Oncol. 23, 251–275 (1996).
Jaffee, E. M., Hruban, R. H., Canto, M. & Kern, S. E. Focus on pancreas cancer. Cancer Cell 2, 25–28 (2002).
Eberle, M. A. et al. A new susceptibility locus for autosomal dominant pancreatic cancer maps to chromosome 4q32-34. Am. J. Hum. Genet. 70, 1044–1048 (2002). Linkage mapping of a new familial pancreatic cancer gene.
Lowenfels, A. B. et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl Cancer Inst. 89, 442–446 (1997).
Whitcomb, D. C. et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature Genet. 14, 141–145 (1996).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Cubilla, A. L. & Fitzgerald, P. J. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 36, 2690–2698 (1976). A landmark study providing histological evidence for a ductal cell of origin for pancreatic adenocarcinoma.
Klimstra, D. S. & Longnecker, D. S. K-ras mutations in pancreatic ductal proliferative lesions. Am. J. Pathol. 145, 1547–1550 (1994).
Hruban, R. H. et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 25, 579–586 (2001).
Klein, W. M., Hruban, R. H., Klein-Szanto, A. J. & Wilentz, R. E. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod. Pathol. 15, 441–447 (2002).
Moskaluk, C. A., Hruban, R. H. & Kern, S. E. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 57, 2140–2143 (1997).
Yamano, M. et al. Genetic progression and divergence in pancreatic carcinoma. Am. J. Pathol. 156, 2123–2133 (2000).
Luttges, J. et al. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am. J. Pathol. 158, 1677–1683 (2001). References 16–18 document common mutational profiles in PanINs and pancreatic adenocarcinomas occurring in the same patient, providing genetic evidence that PanINs are progenitors of adenocarcinomas.
Wilentz, R. E. et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 60, 2002–2006 (2000).
Heinmoller, E. et al. Molecular analysis of microdissected tumors and preneoplastic intraductal lesions in pancreatic carcinoma. Am. J. Pathol. 157, 83–92 (2000).
Rozenblum, E. et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 57, 1731–1734 (1997). Mutational profile of a large series of pancreatic adenocarcinomas.
Biankin, A. V. et al. Overexpression of p21(WAF1/CIP1) is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 61, 8830–8837 (2001).
Shields, J. M., Pruitt, K., McFall, A., Shaub, A. & Der, C. J. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 10, 147–154 (2000).
Korc, M. et al. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J. Clin. Invest. 90, 1352–1360 (1992).
Barton, C. M., Hall, P. A., Hughes, C. M., Gullick, W. J. & Lemoine, N. R. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J. Pathol. 163, 111–116 (1991).
Friess, H. et al. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J. Mol. Med. 74, 35–42 (1996).
Watanabe, M., Nobuta, A., Tanaka, J. & Asaka, M. An effect of K-ras gene mutation on epidermal growth factor receptor signal transduction in PANC-1 pancreatic carcinoma cells. Int. J. Cancer 67, 264–268 (1996).
Sibilia, M. et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102, 211–220 (2000).
Day, J. D. et al. Immunohistochemical evaluation of HER-2/ neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum. Pathol. 27, 119–124 (1996).
Wagner, M. et al. Expression of a truncated EGF receptor is associated with inhibition of pancreatic cancer cell growth and enhanced sensitivity to cisplatinum. Int. J. Cancer 68, 782–787 (1996).
Overholser, J. P., Prewett, M. C., Hooper, A. T., Waksal, H. W. & Hicklin, D. J. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer 89, 74–82 (2000).
Whelan, A. J., Bartsch, D. & Goodfellow, P. J. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N. Engl. J. Med. 333, 975–977 (1995).
Goldstein, A. M. et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N. Engl. J. Med. 333, 970–974 (1995).
Goldstein, A. M., Struewing, J. P., Chidambaram, A., Fraser, M. C. & Tucker, M. A. Genotype-phenotype relationships in U. S. melanoma-prone families with CDKN2A and CDK4 mutations. J. Natl Cancer Inst. 92, 1006–1010 (2000).
Lynch, H. T. et al. Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: the familial atypical mole melanoma-pancreatic carcinoma syndrome. Cancer 94, 84–96 (2002).
Borg, A. et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J. Natl Cancer Inst. 92, 1260–1266 (2000).
Sherr, C. J. The INK4A/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001).
Liu, L. et al. Mutation of the CDKN2A 5′ UTR creates an aberrant initiation codon and predisposes to melanoma. Nature Genet. 21, 128–132 (1999).
Lal, G. et al. Patients with both pancreatic adenocarcinoma and melanoma may harbor germline CDKN2A mutations. Genes Chromosom. Cancer 27, 358–361 (2000).
Krimpenfort, P., Quon, K. C., Mooi, W. J., Loonstra, A. & Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413, 83–86 (2001).
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001). References 40 and 41 report the phenotypes of Ink4a-knockout mice.
Zindy, F., Quelle, D. E., Roussel, M. F. & Sherr, C. J. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15, 203–211 (1997).
Nielsen, G. P. et al. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab. Invest. 79, 1137–1143 (1999).
Sherr, C. J. & DePinho, R. A. Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410 (2000).
Ramirez, R. D. et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15, 398–403 (2001).
Schmitt, C. A. et al. A senescence program controlled by p53 and p16(INK4a) contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002).
Zhu, J., Woods, D., McMahon, M. & Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998).
Brookes, S. et al. INK4A-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21, 2936–2945 (2002).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997). References 47–49 provide an explanation for the oncogenic cooperation of activated RAS genes and loss of the INK4A /ARF locus.
Luttges, J. et al. The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer 85, 1703–1710 (1999).
Laghi, L. et al. Common occurrence of multiple K-RAS mutations in pancreatic cancers with associated precursor lesions and in biliary cancers. Oncogene 21, 4301–4306 (2002).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Chin, L. et al. Cooperative effects of INK4A and RAS in melanoma susceptibility in vivo. Genes Dev. 11, 2822–2834 (1997).
Fisher, G. H. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001).
Sharpless, N. E. & DePinho, R. A. The INK4A/ARF locus and its two gene products. Curr. Opin. Genet. Dev. 9, 22–30 (1999).
Maser, R. S. & DePinho, R. A. Connecting chromosomes, crisis, and cancer. Science 297, 565–569 (2002).
Gorunova, L. et al. Cytogenetic analysis of pancreatic carcinomas: intratumor heterogeneity and nonrandom pattern of chromosome aberrations. Genes Chromosom. Cancer 23, 81–99 (1998).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Gisselsson, D. et al. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc. Natl Acad. Sci. USA 97, 5357–5362 (2000).
Gisselsson, D. et al. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc. Natl Acad. Sci. USA 98, 12683–12688 (2001). Evidence for a role of telomere attrition in promoting chromosomal instability in the progression of pancreatic adenocarcinoma.
Suehara, N. et al. Telomerase elevation in pancreatic ductal carcinoma compared to nonmalignant pathological states. Clin. Cancer Res. 3, 993–998 (1997).
Venkitaraman, A. R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108, 171–182 (2002).
Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J. Natl Cancer Inst. 91, 1310–1316 (1999).
Goggins, M., Hruban, R. H. & Kern, S. E. BRCA2 is inactivated late in the development of pancreatic intraepithelial neoplasia: evidence and implications. Am. J. Pathol. 156, 1767–1771 (2000).
Sato, N. et al. Correlation between centrosome abnormalities and chromosomal instability in human pancreatic cancer cells. Cancer Genet. Cytogenet. 126, 13–19 (2001).
Aarnio, M., Mecklin, J. P., Aaltonen, L. A., Nystrom-Lahti, M. & Jarvinen, H. J. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int. J. Cancer 64, 430–433 (1995).
Goggins, M. et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am. J. Pathol. 152, 1501–1507 (1998).
Mahlamaki, E. H. et al. Comparative genomic hybridization reveals frequent gains of 20q, 8q, 11q, 12p, and 17q, and losses of 18q, 9p, and 15q in pancreatic cancer. Genes Chromosom. Cancer 20, 383–391 (1997).
Peltomaki, P. & de la Chapelle, A. Mutations predisposing to hereditary nonpolyposis colorectal cancer. Adv. Cancer Res. 71, 93–119 (1997).
Lynch, H. T., Voorhees, G. J., Lanspa, S. J., McGreevy, P. S. & Lynch, J. F. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br. J. Cancer 52, 271–273 (1985).
Yamamoto, H. et al. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 61, 3139–3144 (2001).
Wilentz, R. E. et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: a newly described and characterized entity. Am. J. Pathol. 156, 1641–1651 (2000).
Hahn, S. A. et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271, 350–353 (1996). Identification of SMAD4/DPC4.
Massague, J., Blain, S. W. & Lo, R. S. TGF-β signaling in growth control, cancer, and heritable disorders. Cell 103, 295–309 (2000).
Sirard, C. et al. Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor beta-related signaling. J. Biol. Chem. 275, 2063–2070 (2000).
Jonson, T. et al. Altered expression of TGF-β receptors and mitogenic effects of TGF-β in pancreatic carcinomas. Int. J. Oncol. 19, 71–81 (2001).
Dai, J. L. et al. Transforming growth factor-beta responsiveness in DPC4/SMAD4-null cancer cells. Mol. Carcinog. 26, 37–43 (1999).
Giehl, K., Seidel, B., Gierschik, P., Adler, G. & Menke, A. TGF-β1 represses proliferation of pancreatic carcinoma cells which correlates with Smad4-independent inhibition of ERK activation. Oncogene 19, 4531–4541 (2000).
Rowland-Goldsmith, M. A., Maruyama, H., Kusama, T., Ralli, S. & Korc, M. Soluble type II transforming growth factor-beta (TGF-beta) receptor inhibits TGF-beta signaling in COLO-357 pancreatic cancer cells in vitro and attenuates tumor formation. Clin. Cancer Res. 7, 2931–2940 (2001).
Hemminki, A. et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391, 184–187 (1998).
Solcia, E., Capella, C. & Kloppel, G. Tumors of the Pancreas (ed. Rosai, J.) (Armed Forces Institute for Pathology, Washington DC, 1995).
Pour, P. M. The role of langerhans islets in pancreatic ductal adenocarcinoma. Front Biosci. 2, d271–282 (1997).
Boardman, L. A. et al. Genetic heterogeneity in Peutz–Jeghers syndrome. Hum. Mutat. 16, 23–30 (2000).
Cooper, H. S. in Pathology of the Gastrointestinal Tract (eds Ming, S.-C. & Goldman, H.) 819–853 (Wiliams & Wilkens, Baltimore, 1998).
Olschwang, S. et al. Peutz–Jeghers disease: most, but not all, families are compatible with linkage to 19p13.3. J. Med. Genet. 35, 42–44 (1998).
Olschwang, S., Boisson, C. & Thomas, G. Peutz–Jeghers families unlinked to STK11/LKB1 gene mutations are highly predisposed to primitive biliary adenocarcinoma. J. Med. Genet. 38, 356–360 (2001).
Klimstra, D. S. in Pancreatic Cancer: Advances in Molecular Pathology, Diagnosis and Clinical Management (eds Sarkar, F. S. & Duggan, M. C.) 21–48 (Eaton Publishing, Natick, Massachusetts, 1998).
Jimenez, R. E. et al. Immunohistochemical characterization of pancreatic tumors induced by dimethylbenzanthracene in rats. Am. J. Pathol. 154, 1223–1229 (1999).
Wagner, M. et al. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 15, 286–293 (2001). The first description of a genetically defined mouse model of pancreatic adenocarcinoma.
Yoshida, T. & Hanahan, D. Murine pancreatic ductal adenocarcinoma produced by in vitro transduction of polyoma middle T oncogene into the islets of Langerhans. Am. J. Pathol. 145, 671–684 (1994).
Tosh, D. & Slack, J. M. How cells change their phenotype. Nature Rev. Mol. Cell Biol. 3, 187–194 (2002).
Blau, H. M., Brazelton, T. R. & Weimann, J. M. The evolving concept of a stem cell: entity or function? Cell 105, 829–841 (2001).
Bonner–Weir, S. & Sharma, A. Pancreatic stem cells. J. Pathol. 197, 519–526 (2002).
Elsasser, H.-P., Adler, G. & Kern, H. F. in The Pancreas: Biology, Pathobiology and Disease (Raven Press Ltd, New York, 1993).
Bonner–Weir, S., Stubbs, M., Reitz, P., Taneja, M. & Smith, F. E. in Pancreatic Growth and Regeneration (ed. Sarvetnick, N.) (Karger Landes Systems, Basel, Switzerland, 1997).
Sharma, A. et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48, 507–513 (1999).
Vinik, A. I., Pittenger, G. L., Rafaeloff, R., Rosenberg, L. & Duguid, W. in Pancreatic Growth and Regeneration. (ed. Sarvetnick, N.) 183–217 (Karger Landes Systems, Basel, 1997).
Scoggins, C. R. et al. p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G827–G836 (2000).
Kritzik, M. R. et al. PDX-1 and Msx-2 expression in the regenerating and developing pancreas. J. Endocrinol. 163, 523–530 (1999).
Arnush, M. et al. Growth factors in the regenerating pancreas of γ-interferon transgenic mice. Lab. Invest. 74, 985–990 (1996).
Rooman, I., Heremans, Y., Heimberg, H. & Bouwens, L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 43, 907–914 (2000).
Bachoo, R. M. et al. Epidermal growth factor receptor and Ink4a/Arf. Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1, 269–277 (2002).
Lohr, M. et al. Transforming growth factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 61, 550–555 (2001).
Schwarte-Waldhoff, I. et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc. Natl Acad. Sci. USA 97, 9624–9629 (2000).
Bissell, M. J. & Radisky, D. Putting tumours in context. Nature Rev. Cancer 1, 46–54 (2001).
Olumi, A. F. et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 59, 5002–5011 (1999).
Van Dyke, T. & Jacks, T. Cancer modeling in the modern era: progress and challenges. Cell 108, 135–144 (2002).
Ornitz, D. M., Hammer, R. E., Messing, A., Palmiter, R. D. & Brinster, R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science 238, 188–193 (1987).
Glasner, S., Memoli, V. & Longnecker, D. S. Characterization of the ELSV transgenic mouse model of pancreatic carcinoma. Histologic type of large and small tumors. Am. J. Pathol. 140, 1237–1245 (1992).
Quaife, C. J., Pinkert, C. A., Ornitz, D. M., Palmiter, R. D. & Brinster, R. L. Pancreatic neoplasia induced by Ras expression in acinar cells of transgenic mice. Cell 48, 1023–1034 (1987).
Sandgren, E. P., Quaife, C. J., Paulovich, A. G., Palmiter, R. D. & Brinster, R. L. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci USA 88, 93–97 (1991).
Sandgren, E. P. et al. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol. Cell Biol. 13, 320–330 (1993).
Bardeesy, N. et al. Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol. Cell Biol. 22, 635–643 (2002).
Sotillo, R. et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20, 6637–6647 (2001).
Rane, S. G. et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nature Genet. 22, 44–52 (1999).
Xu, X. et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19, 1868–1874 (2000).
Takaku, K. et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 59, 6113–6117 (1999).
Jishage, K. et al. Role of Lkb1, the causative gene of Peutz–Jegher's syndrome, in embryogenesis and polyposis. Proc. Natl Acad. Sci. USA 99, 8903–8908 (2002).
Miyoshi, H. et al. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 62, 2261–2266 (2002).
Bardeesy, N. et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419, 162–167 (2002).
Robanus-Maandag, E. et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 12, 1599–1609 (1998).
Jonkers, J. & Berns, A. Conditional mouse models of sporadic cancer. Nature Rev. Cancer 2, 251–265 (2002).
Gu, G., Dubauskaite, J. & Melton, D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Hennig, R. et al. 5-lipoxygenase and leukotriene b(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am. J. Pathol. 161, 421–428 (2002).
Maitra, A. et al. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am. J. Clin. Pathol. 118, 194–201 (2002).
Tucker, O. N. et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 59, 987–990 (1999).
Anderson, K. E., Johnson, T. W., Lazovich, D. & Folsom, A. R. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J. Natl Cancer Inst. 94, 1168–1171 (2002).
Oshima, M. & Taketo, M. M. COX selectivity and animal models for colon cancer. Curr. Pharm. Des. 8, 1021–1034 (2002).
Ramaswamy, S. & Golub, T. R. DNA microarrays in clinical oncology. J. Clin. Oncol. 20, 1932–1941 (2002).
Argani, P. et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 61, 4320–4324 (2001).
Iacobuzio-Donahue, C. A. et al. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am. J. Pathol. 160, 1239–1249 (2002).
Rosty, C. et al. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 62, 1868–1875 (2002).
Han, H. et al. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 62, 2890–2896 (2002).
Githens, S. in The Pancreas: Biology, Pathobiology and Disease (eds Liang, V. & Go, W.) 21–55 (Raven Press Ltd, New York, 1993).
Slack, J. M. Developmental biology of the pancreas. Development 121, 1569–1580 (1995).
Kim, S. K. & Hebrok, M. Intercellular signals regulating pancreas development and function. Genes Dev. 15, 111–127 (2001).
Kobitsu, K. et al. Shortened telomere length and increased telomerase activity in hamster pancreatic duct adenocarcinomas and cell lines. Mol. Carcinog. 18, 153–159 (1997).
Edlund, H. Organogenesis: pancreatic organogenesis developmental mechanisms and implications for therapy. Nature Rev. Genet. 3, 524–532 (2002).
Hebrok, M., Kim, S. K. & Melton, D. A. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 12, 1705–1713 (1998).
Wells, J. M. & Melton, D. A. Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 15, 393–410 (1999).
Shen, C. N., Slack, J. M. & Tosh, D. Molecular basis of transdifferentiation of pancreas to liver. Nature Cell Biol. 2, 879–887 (2000).
Harada, T. et al. Interglandular cytogenetic heterogeneity detected by comparative genomic hybridization in pancreatic cancer. Cancer Res 62, 835–839 (2002).
Giardiello, F. M. et al. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 119, 1447–1453 (2000).
Clarke, A. R., Cummings, M. C. & Harrison, D. J. Interaction between murine germline mutations in p53 and APC predisposes to pancreatic neoplasia but not to increased intestinal malignancy. Oncogene 11, 1913–1920 (1995).
Meszoely, I. M., Means, A. L., Scoggins, C. R. & Leach, S. D. Developmental aspects of early pancreatic cancer. Cancer J. 7, 242–250 (2001).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
OMIM
FURTHER INFORMATION
Glossary
- PANCREATIC ADENOCARCINOMA
-
The most common type of cancer of the pancreas, accounting for greater than 85% of pancreatic neoplasms. These tumours show histological resemblance to the pancreatic ducts and are also referred to as pancreatic ductal adenocarcinoma, or simply pancreatic cancer.
- PANCREATICODUODENECTOMY
-
A surgical resection of the distal stomach, duodenum, common duct, and head of the pancreas, containing the pancreatic neoplasm.
- INK4A
-
A cyclin-dependent kinase inhibitor that acts on cyclin-D–CDK4/6. It prevents hyperphosphorylation and inactivation of RB, so cells can not enter S phase.
- ARF
-
A regulator of p53 that functions by inhibiting MDM2-dependent proteolysis of p53.
- LOSS OF HETEROZYGOSITY
-
(LOH). In cells that carry a mutated allele of a tumour-suppressor gene, the gene becomes fully inactivated when the cell loses a large part of the chromosome carrying the wild-type allele. Regions with high frequency of LOH are believed to harbour tumour-suppressor genes.
- MAPK PATHWAY
-
A signal-transduction pathway that is crucial for the integration of mitogenic signals mediated by various growth factors. Activation of this pathway is involved in many cellular processes, including cell-cycle progression.
- AUTOCRINE SIGNALLING
-
A signal-transduction mechanism in which both a secreted ligand and corresponding cell-surface receptor are expressed by the same cell.
- PI3K PATHWAY
-
The phosphatidylinositol 3-kinase (PI3K) family of enzymes are activated in response to various stimuli and catalyse the phophorylation of inositol lipids at the d-3 position of the inositol ring. These phosphoinositides act as second messengers; a primary target is the serine/threonine kinase AKT (protein kinase B). Activated AKT phosphorylates several cellular targets, including proteins that are involved in cell survival, proliferation and migration.
- FIELD DEFECT
-
The independent transformation of different epithelial cells following exposure of an area of tissue to carcinogenic insults.
- COMPARATIVE GENOMIC HYBRIDIZATION
-
A method that is used to detect chromosome copy-number alterations. Tumour and control DNA are labelled with different coloured fluorescent dyes and hybridized to metaphase chromosomes. Deletions/amplifications are visualized by changes in the fluorescent spectrum at specific chromosomal regions. Array-CGH allows higher resolution and accuracy as it uses hybridization to small DNA fragments that are arranged on microchips.
- TRANSFORMING GROWTH FACTOR-β FAMILY
-
Secreted growth factors, comprising the TGF-β, bone morphogenic protein and activin families, which transduce signals through specific cell-surface receptors. These signalling pathways control many morphogenic and homeostatic processes. SMAD4 mediates TGF-β-family signalling, linking receptor stimulation to transcriptional responses by complexing with specific receptor-activated SMADs and other transcriptional components.
- STROMA
-
The non-transformed component of epithelial tumours, comprised of fibroblasts, blood-vessel elements and immune cells. The stromal cells do not show the same clonal genetic changes as the malignant tumour cells, yet are altered by molecular events in the tumour cells and, in turn, modulate biological features of the tumour compartment. These heterotypic interactions are fundamental to tumour genesis and progression.
- CELL OF ORIGIN
-
The cell type and specific developmental stage from which a tumour type evolves. Although epithelial tumours are classified by their histological resemblance to normal cellular counterparts, this might not strictly indicate a cell-lineage relationship, given the known developmental plasticity of malignant cells. Understanding the cell of origin in pancreatic adenocarcinoma might help in prevention, diagnosis and treatment.
- TRANSDIFFERENTIATION
-
The conversion of one differentiated cell type into another. Transdifferentiation and the conversion between undifferentiated stem cells of different tissue are subclasses of metaplasia.
- APC Min/+
-
A mouse strain that is prone to the development of multiple intestinal polyps, due to mutations in the Apc gene — the homologue of the human familial colon cancer gene.
- CRE RECOMBINASE
-
An enzyme that is derived from the P1 bacteriophage that binds to a 34-base-pair DNA sequence (loxP site). Sequences flanked by loxP sites can be excised by Cre recombinase expression. The Cre–Lox system is a technology that allows genes to be ablated in a tissue-selective and inducible manner.
- AVIAN RETROVIRAL RECEPTOR
-
An avian cell-surface protein that is required for infection by the avian sarcoma and leukosis virus (ASLV). Tissue-specific expression of the gene encoding this receptor in transgenic mice enables infection of these tissues with ASLV, allowing somatically targeted gene transfer.
Rights and permissions
About this article
Cite this article
Bardeesy, N., DePinho, R. Pancreatic cancer biology and genetics. Nat Rev Cancer 2, 897–909 (2002). https://doi.org/10.1038/nrc949
Issue Date:
DOI: https://doi.org/10.1038/nrc949
This article is cited by
-
Optimization of a mouse model of pancreatic cancer to simulate the human phenotypes of metastasis and cachexia
BMC Cancer (2024)
-
An Overview on the Emerging Role of the Plasma Protease Inhibitor Protein ITIH5 as a Metastasis Suppressor
Cell Biochemistry and Biophysics (2024)
-
A novel immune checkpoint score system for prognostic evaluation in pancreatic adenocarcinoma
BMC Gastroenterology (2023)
-
PKCι induces differential phosphorylation of STAT3 to modify STAT3-related signaling pathways in pancreatic cancer cells
Journal of Cell Communication and Signaling (2023)
-
Therapeutic potential of chrysin nanoparticle-mediation inhibition of succinate dehydrogenase and ubiquinone oxidoreductase in pancreatic and lung adenocarcinoma
European Journal of Medical Research (2022)