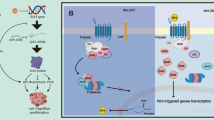
Key Points
Summary
-
Although deregulated homeobox gene expression was originally associated with oncogenic activities, it is now apparent that homeobox genes might be lost as well as gained in cancer, and their corresponding activities might be tumour suppressing as well as tumour promoting. Although there are numerous examples of the deregulated expression of homeobox genes in association with cancer, there are far fewer cases of causal links between these aberrant expressions and carcinogenesis.
-
Many examples of deregulated homeobox gene expression in cancer conform to a simple rule: homeobox genes that are upregulated in cancer are normally expressed during development and/or in undifferentiated cells, whereas homeobox genes that are downregulated in cancer are normally expressed in adulthood and/or in differentiated tissues.
-
Several examples exist in which homeobox genes impinge on the cell cycle, indicating that deregulation of cell-cycle control might be a common mechanism by which aberrant homeobox gene expression contributes to carcinogenesis.
-
Homeobox genes that are downregulated in cancer share several features, including tissue specificity and epigenetic loss of function.
-
Based on their unique features with respect to their expression and function, homeobox genes are best described as 'tumour modulators' rather than oncogenes or tumour-suppressor genes.
Abstract
Homeobox genes comprise a large and essential family of developmental regulators that are vital for all aspects of growth and differentiation. Although many studies have reported their deregulated expression in cancer, few studies have established direct functional roles for homeobox genes in carcinogenesis. Nonetheless, most cases of deregulated homeobox gene expression in cancer conform to a simple rule: those that are normally expressed in undifferentiated cells are upregulated in cancer, whereas those that are normally expressed in differentiated tissues are downregulated in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Duboule, D. (ed.) Guidebook to the Homeobox Genes (Oxford Univ. Press, New York, 1994).
Stein, S., Fritsch, R., Lemaire, L. & Kessel, M. Checklist: vertebrate homeobox genes. Mech. Dev. 55, 91–108 (1996).
Tupler, R., Perini, G. & Green, M. R. Expressing the human genome. Nature 409, 832–833 (2001).
Krumlauf, R. Hox genes in vertebrate development. Cell 78, 191–201 (1994).
Boncinelli, E., Mallamaci, A. & Lavorgna, G. Vertebrate homeobox genes. Genetica 94, 127–140 (1994).
Maconochie, M., Nonchev, S., Morrison, A. & Krumlauf, R. Paralogous Hox genes: function and regulation. Ann. Rev. Genet. 30, 529–556 (1996).
Duboule, D. Vertebrate Hox genes and proliferation: an alternative pathway to homeosis? Curr. Opin. Genet. Dev. 5, 525–528 (1995).
Chen, F. & Capecchi, M. R. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc. Natl Acad. Sci. USA 96, 541–546 (1999).
Davidson, D. The function and evolution of Msx genes: pointers and paradoxes. Trends Genet. 11, 405–411 (1995).
Bendall, A. J. & Abate-Shen, C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 247, 17–31 (2000).
Song, K., Wang, Y. & Sassoon, D. Expression of Hox-7.1 in myoblasts inhibits terminal differentiation and induces cell transformation. Nature 360, 477–481 (1992).Shows that overpression of Msx1 (formerly Hox-7.1 ) in cell culture blocks differentiation and promotes a transformed phenotype.
Woloshin, P. et al. MSX1 inhibits myoD expression in fibroblast x 10T1/2 cell hybrids. Cell 82, 611–620 (1995).
Mina, M., Gluhak, J. & Rodgers, B. Downregulation of Msx-2 expression results in chondrogenesis in the medial region of the avian mandible. Connect. Tissue Res. 35, 79–84 (1996).
Dodig, M. et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev. Biol. 209, 298–307 (1999).
Hu, G., Lee, H., Price, S. M., Shen, M. M. & Abate-Shen, C. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128, 2373–2384 (2001).Proposes that inhibition of differentiation by Msx1 is a consequence of its ability to upregulate cyclin D1, which, in turn, prevents exit from the cell cycle.
Bhatia-Gaur, R. et al. Roles for Nkx3. 1 in prostate development and cancer. Genes Dev. 13, 966–977 (1999).Demonstrates that Nkx3.1 -mutant mice are predisposed to prostate carcinoma; implicates the loss of function of this homeobox gene as a causal factor in prostate carcinogenesis.
Damante, G., Tell, G. & Di Lauro, R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog. Nucleic Acid Res. Mol. Biol. 66 307–356 (2001).
Harvey, R. P. NK-2 homeobox genes and heart development. Dev. Biol. 178, 203–216 (1996).
Maconochie, M. K. et al. Cross-regulation in the mouse HoxB complex: the expression of Hoxb2 in rhombomere 4 is regulated by Hoxb1. Genes Dev. 11, 1885–1895 (1997).
Ferretti, E. et al. Segmental expression of Hoxb2 in r4 requires two separate sites that integrate cooperative interactions between Prep1, Pbx and Hox proteins. Development 127, 155–166 (2000).
Livesey, F. J., Furukawa, T., Steffen, M. A., Church, G. M. & Cepko, C. L. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Curr. Biol. 10, 301–310 (2000).
Mann, R. S. & Chan, S. K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 12, 258–262 (1996).
Mann, R. S. & Affolter, M. Hox proteins meet more partners. Curr. Opin. Genet. Dev. 8, 423–429. (1998).
Rieckhof, G. E., Casares, F., Ryoo, H. D., Abu-Shaar, M. & Mann, R. S. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171–183 (1997).
Abu-Shaar, M., Ryoo, H. D. & Mann, R. S. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 13, 935–945 (1999).
Biggin, M. D. & McGinnis, W. Regulation of segmentation and segmental identity by Drosophila homeoproteins: the role of DNA binding in functional activity and specificity. Development 124, 4425–4433 (1997).
Peverali, F. A. et al. Expression of HOX homeogenes in human neuroblastoma cell culture lines. Differentiation 45, 61–69 (1990).
Cillo, C. et al. HOX gene expression in normal and neoplastic human kidney. Int. J. Cancer 51, 892–897 (1992).One of the first studies to provide a comprehensive survey of Hox gene expression in cancer.
Cillo, C., Cantile, M., Faiella, A. & Boncinelli, E. Homeobox genes in normal and malignant cells. J. Cell. Physiol. 188, 161–169 (2001).
Kozmik, Z., Sure, U., Ruedi, D., Busslinger, M. & Aguzzi, A. Deregulated expression of PAX5 in medulloblastoma. Proc. Natl Acad. Sci. USA 92, 5709–5713 (1995).
Bowen, C. et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 60, 6111–6115 (2000).
Ee, H. C., Erler, T., Bhathal, P. S., Young, G. P. & James, R. J. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am. J. Pathol. 147, 586–592 (1995).
Aberdam, D., Negreanu, V., Sachs, L. & Blatt, C. The oncogenic potential of an activated Hox-2.4 homeobox gene in mouse fibroblasts. Mol. Cell. Biol. 11, 554–557 (1991).One of the first papers to demonstrate the oncogenic potential of homeobox genes.
Maulbecker, C. C. & Gruss, P. The oncogenic potential of deregulated homeobox genes. Cell Growth Differ 4, 431–441 (1993).Shows that overexpression of several homeobox genes promote tumorigenesis. One of the first studies to suggest that the generality of deregulated homeobox genes indicate expression as a causal role in carcinoma.
Maulbecker, C. C. & Gruss, P. The oncogenic potential of Pax genes. EMBO J. 12, 2361–2367 (1993).
Takahashi, C. et al. Characterization of a human MSX-2 cDNA and its fragment isolated as a transformation suppressor gene against v-Ki-ras oncogene. Oncogene 12, 2137–2146 (1996).
Takahashi, C., Akiyama, N., Kitayama, H., Takai, S. & Noda, M. Possible involvement of MSX-2 homeoprotein in v-ras-induced transformation. Leukemia 11 340–343 (1997).
Care, A. et al. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol. Cell. Biol. 16, 4842–4851 (1996).
Naora, H. et al. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. Proc. Natl Acad. Sci. USA 98, 4060–4065 (2001).
Hall, M. & Peters, G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv. Cancer Res. 68, 67–108 (1996).
Suzuki, M. et al. Over-expression of HOX-8, the human homologue of the mouse Hox-8 homeobox gene, in human tumors. Biochem. Biophys. Res. Commun. 194, 187–193 (1993).
Ford, H. L., Kabingu, E. N., Bump, E. A., Mutter, G. L. & Pardee, A. B. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc. Natl Acad. Sci. USA 95, 12608–12613 (1998).Demonstrates that overexpression of HSIX1 promotes tumorigenesis by abrogating the G2 cell-cycle checkpoint.
Ford, H. L. et al. Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J. Biol. Chem. 275, 22245–22254 (2000).
Kawabe, T., Muslin, A. J. & Korsmeyer, S. J. HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature 385, 454–458 (1997).Proposes that the oncogenic potential of HOX11 is mediated by altering the cell cycle, which is a consequence of its interaction with protein phosphatases that regulate the G2/M cell-cycle checkpoint. One of the first examples showing that homeobox genes can promote oncogenesis by perturbing cell-cycle control.
Hatano, M., Roberts, C. W., Minden, M., Crist, W. M. & Korsmeyer, S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukaemia. Science 253, 79–82 (1991).
Kennedy, M. A. et al. HOX11, a homeobox-containing T-cell oncogene on human chromosome 10q24. Proc. Natl Acad. Sci. USA 88, 8900–8904 (1991).
Dube, I. D. et al. A novel human homeobox gene lies at the chromosome 10 breakpoint in lymphoid neoplasias with chromosomal translocation t(10;14). Blood 78, 2996–3003 (1991).
Lu, M., Gong, Z. Y., Shen, W. F. & Ho, A. D. The tcl-3 proto-oncogene altered by chromosomal translocation in T-cell leukaemia codes for a homeobox protein. EMBO J. 10, 2905–2910 (1991).
Dear, T. N. et al. The Hox11 gene is essential for cell survival during spleen development. Development 121, 2909–2915 (1995).
Raman, V. et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature 405, 974–978 (2000).
Smith, R. C. et al. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 11, 1674–1689 (1997).Shows that overexpression of GAX leads to upregulation of WAF1, which, in turn, leads to cell-cycle arrest.
James, R. & Kazenwadel, J. Homeobox gene expression in the intestinal epithelium of adult mice. J. Biol. Chem. 266, 3246–3251 (1991).
James, R., Erler, T. & Kazenwadel, J. Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J. Biol. Chem. 269, 15229–15237 (1994).
Silberg, D. G., Swain, G. P., Suh, E. R. & Traber, P. G. Cdx1 and cdx2 expression during intestinal development. Gastroenterology 119, 961–971 (2000).
Mallo, G. V. et al. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int. J. Cancer 74, 35–44 (1997).
Hinoi, T. et al. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am. J. Pathol. 159, 2239–2248 (2001).
Chawengsaksophak, K., James, R., Hammond, V. E., Kontgen, F. & Beck, F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 386, 84–87 (1997).Demonstrates that Cdx2 heterozygous mutant mice develop precancerous lesions of the intestine that are coincident with loss of Cdx2 protein expression. One of the first studies to associate loss (rather than gain) of function of a homeobox gene with carcinogenesis.
Mallo, G. V. et al. Expression of the Cdx1 and Cdx2 homeotic genes leads to reduced malignancy in colon cancer-derived cells. J. Biol. Chem. 273, 14030–14036 (1998).
Suh, E. & Traber, P. G. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol. Cell. Biol. 16, 619–625 (1996).
Tamai, Y. et al. Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res. 59, 2965–2970 (1999).
Sivagnanasundaram, S. et al. The homeobox gene CDX2 in colorectal carcinoma: a genetic analysis. Br. J. Cancer 84, 218–25 (2001).
Wicking, C. et al. CDX2, a human homologue of Drosophila caudal, is mutated in both alleles in a replication error positive colorectal cancer. Oncogene 17, 657–659 (1998).
Bieberich, C. J., Fujita, K., He, W. W. & Jay, G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J. Biol. Chem. 271, 31779–31782 (1996).
He, W. W. et al. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics 43, 69–77 (1997).
Prescott, J. L., Blok, L. & Tindall, D. J. Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate 35, 71–80 (1998).
Sciavolino, P. J. & Abate-Shen, C. Molecular biology of prostate development and prostate cancer. Ann. Med. 30, 357–368 (1998).
Tanaka, M. et al. Nkx3.1, a murine homolog of Drosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev. Dyn. 219, 248–260 (2000).
Kim, M. J. et al. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 62, 2999–3004 (2002)
Abdulkadir, S. A. et al. Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol. Cell. Biol. 22, 1495–1503 (2002).
Kim, M. J. et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl Acad. Sci. USA 99, 2884–2889 (2002).
Voeller, H. J. et al. Coding region of NKX3.1, a prostate-specific homeobox gene on 8p21, is not mutated in human prostate cancers. Cancer Res. 57, 4455–4459 (1997).
Vocke, C. D. et al. Analysis of 99 microdissected prostate carcinomas reveals a high frequency of allelic loss on chromosome 8p12-21. Cancer Res. 56, 2411–2416 (1996).
Emmert-Buck, M. R. et al. Allelic loss on chromosome 8p12–21 in microdissected prostati intraepithelial neoplasia. Cancer Res. 55, 2959–2962 (1995).
Lawrence, H. J., Sauvageau, G., Humphries, R. K. & Largman, C. The role of HOX homeobox genes in normal and leukemic hematopoiesis. Stem Cells 14, 281–291 (1996).
Look, A. T. Oncogenic transcription factors in the human acute leukemias. Science 278, 1059–1064 (1997).
van Oostveen, J., Bijl, J., Raaphorst, F., Walboomers, J. & Meijer, C. The role of homeobox genes in normal hematopoiesis and hematological malignancies. Leukemia 13, 1675–1690 (1999).
Quon, K. C. & Berns, A. Haplo-insufficiency? Let me count the ways. Genes Dev. 15, 2917–2921 (2001).
Barr, F. G. The role of chimeric paired box transcription factors in the pathogenesis of paediatric rhabdomysarcoma. Cancer Res. 59, 1711s–1715s (1999).
Barr, F. G. et al. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nature Genet. 3, 113–117 (1993).
Davis, R. J., D'Cruz, C. M., Lovell, M. A., Biegel, J. A. & Barr, F. G. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 54, 2869–2872 (1994).
Lam, P. Y., Sublett, J. E., Hollenbach, A. D. & Roussel, M. F. The oncogenic potential of the Pax3–FKHR fusion protein requires the Pax3 homeodomain recognition helix but not the Pax3 paired-box DNA binding domain. Mol. Cell. Biol. 19, 594–601 (1999).
Kamps, M. P. & Baltimore, D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukaemia, causes acute myeloid leukaemia in mice. Mol. Cell. Biol. 13, 351–357 (1993).
Nourse, J. et al. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell 60, 535–545 (1990).
Borrow, J. et al. The t(7;11)(p15;p15) translocation in acute myeloid leukaemia fuses the genes for nucleoporin NUP98 and class I homeoprotein HOXA9. Nature Genet. 12, 159–167 (1996).
Nakamura, T. et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nature Genet. 12, 154–158 (1996).
Calvo, K. R., Sykes, D. B., Pasillas, M. P. & Kamps, M. P. Nup98-HoxA9 immortalizes myeloid progenitors, enforces expression of Hoxa9, Hoxa7 and Meis1, and alters cytokine-specific responses in a manner similar to that induced by retroviral co-expression of Hoxa9 and Meis1. Oncogene 21, 4247–4256 (2002).
Nakamura, T., Yamazaki, Y., Hatano, Y. & Miura, I. NUP98 is fused to PMX1 homeobox gene in human acute myelogenous leukaemia with chromosome translocation t(1;11)(q23;p15). Blood 94, 741–747 (1999).
Dubrulle, J., McGrew, M. J. & Pourquie, O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106, 219–232 (2001).
Zakany, J., Kmita, M., Alarcon, P., de la Pompa, J. L. & Duboule, D. Localized and transient transcription of Hox genes suggests a link between patterning and the segmentation clock. Cell 106, 207–217 (2001).
Boyl, P. P. et al. Forebrain and midbrain development requires epiblast-restricted Otx2 translational control mediated by its 3′ UTR. Development 128, 2989–3000 (2001).
Friedmann, Y., Daniel, C. A., Strickland, P. & Daniel, C. W. Hox genes in normal and neoplastic mouse mammary gland. Cancer Res. 54, 5981–5985 (1994).
De Vita, G. et al. Expression of homeobox-containing genes in primary and metastatic colorectal cancer. Eur. J. Cancer 6, 887–893 (1993).
Tiberio, C. et al. HOX gene expression in human small-cell lung cancers xenografted into nude mice. Int. J. Cancer 58, 608–615 (1994).
Li, C. M. et al. Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am. J. Pathol. 160, 2181–2190 (2002).
Gao, A. C. & Isaacs, J. T. Expression of homeobox gene-GBX2 in human prostatic cancer cells. Prostate 29, 395–398 (1996).
Gao, A. C., Lou, W. & Isaacs, J. T. Down-regulation of homeobox gene GBX2 expression inhibits human prostate cancer clonogenic ability and tumorigenicity. Cancer Res. 58, 1391–1394 (1998).
Gao, A. C., Lou, W. & Isaacs, J. T. Enhanced GBX2 expression stimulates growth of human prostate cancer cells via transcriptional up-regulation of the interleukin 6 gene. Clin. Cancer Res. 6, 493–497 (2000).
Dressler, G. R. & Douglass, E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Natl Acad. Sci. USA 89, 1179–1183 (1992).
Poleev, A. et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms' tumors. Development 116, 611–623 (1992).
Silberstein, G. B., Dressler, G. R. & Van Horn, K. Expression of the PAX2 oncogene in human breast cancer and its role in progesterone-dependent mammary growth. Oncogene 21, 1009–1016 (2002).
Kroll, T. G. et al. PAX8-PPARγ1 fusion oncogene in human thyroid carcinoma. Science 289, 1357–1360 (2000).
Sellar, G. C. et al. BARX2 induces cadherin 6 expression and is a functional suppressor of ovarian cancer progression. Cancer Res. 61, 6977–6981 (2001).
Acknowledgements
I would like to thank B. McGinnis, A. Rabson, A. Shatkin, M. Shen, R. Stewart, A. Stock and E. White for comments on the manuscript. The diagram of the homeodomain bound to DNA was provided by R. Ebright. Research in my laboratory is supported by the National Cancer Institute and the Department of Defense.
Author information
Authors and Affiliations
Supplementary information
Related links
Related links
DATABASES
Cancer.gov
LocusLink
OMIM
FURTHER INFORMATION
Glossary
- HOMEOBOX
-
An evolutionarily conserved 180-base-pair sequence motif that is located in a large number of known or predicted genes that function as developmental regulators.
- HOMEOPROTEINS
-
The proteins that are encoded by homeobox genes.
- HOMEOTIC GENES
-
Mutations of these Drosophila genes result in homeotic mutations — the conversion of one body part to another. Homeotic genes were identified as part of a hierarchy of genes that control Drosophila development. Other classes of genes in this hierarchy, such as the gap, pair-ruled and segment polarity genes, also include homeobox genes.
- HOMEODOMAIN
-
The 60-amino-acid protein domain encoded by the homeobox.
- PARALOGOUS HOX GENES
-
Refers to genes located in the same position on the four HOX clusters. In general, paralogous HOX genes (for example HOXA9 and HOXB9) are more similar to each other than to adjacent genes on the same HOX cluster (for example, HOXA9 and HOXA10).
- EPITHELIAL–MESENCHYMAL INTERACTIONS
-
Describes the reciprocal signalling between epithelial and mesenchymal tissue layers during embryogenesis. Epithelial–mesenchymal interactions are essential for the formation of many organs.
- ALVEOLAR RHABDOMYOSARCOMA
-
A soft-tissue tumour with muscle differentiation occurring in children.
- PARAXIAL MESODERM
-
The tissue that gives rise to the somites during development.
- BASIC FIBROBLAST GROWTH FACTOR
-
(bFGF). A mitogenic growth factor that is widely utilized during development.
- ADENOMATOUS INTESTINAL POLYP
-
A pre-malignant lesion that is a presumed precursor of colorectal cancer.
- HAPLOINSUFFICIENCY
-
Loss or mutation of a single allele results in a detectable phenotype. Although the classic description of tumour-suppressor genes presumes inactivation of two alleles, there are now several examples in which loss or mutation of a single allele contributes to tumorigenicity77.
- MIN MICE
-
Mice that have a mutation of the tumour-suppressor gene adenomatous polyposis coli (Apc). These mice are prone to develop intestinal polyps.
- EPIGENETIC INACTIVATION
-
Alteration in gene activity that influences the phenotype without affecting the genotype. Examples are promoter methylation and post-transcriptional regulation.
- PROSTATIC INTRAEPITHELIAL NEOPLASIA
-
(PIN). Pre-malignant lesions that are presumed precursors of prostate cancer.
Rights and permissions
About this article
Cite this article
Abate-Shen, C. Deregulated homeobox gene expression in cancer: cause or consequence?. Nat Rev Cancer 2, 777–785 (2002). https://doi.org/10.1038/nrc907
Issue Date:
DOI: https://doi.org/10.1038/nrc907
This article is cited by
-
Retinal determination gene networks: from biological functions to therapeutic strategies
Biomarker Research (2023)
-
TRAF7-targeted HOXA5 acts as a tumor suppressor in prostate cancer progression and stemness via transcriptionally activating SPRY2 and regulating MEK/ERK signaling
Cell Death Discovery (2023)
-
Homeobox B9 Promotes Colon Cancer Progression by Targeting SRSF3
Digestive Diseases and Sciences (2023)
-
Histone H3-wild type diffuse midline gliomas with H3K27me3 loss are a distinct entity with exclusive EGFR or ACVR1 mutation and differential methylation of homeobox genes
Scientific Reports (2023)
-
HOXA13 promotes gastric cancer progression partially via the FN1-mediated FAK/Src axis
Experimental Hematology & Oncology (2022)