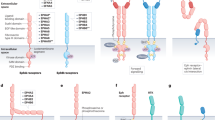
Key Points
-
During the early stages of tumorigenesis, transforming growth factor-β (TGFβ) functions as a tumour suppressor. However, as tumours progress, tumour cells may lose their growth-inhibitory response to TGFβ and may instead respond by initiating epithelial-to-mesenchymal transition and by increasing cell migration.
-
Experimental data support the idea that both loss of and gain of TGFβ signalling is pro-tumorigenic, as mouse models show that the overexpression of TGFβ and the abrogation of signalling results in increased tumour cell metastasis. It is beginning to be appreciated that the effects of TGFβ signalling in the tumour epithelium extend beyond tumour cell-autonomous mechanisms into the tumour microenvironment.
-
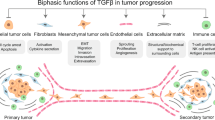
Nearly every cell in the tumour microenvironment responds to TGFβ in a unique way, and these diverse biological responses have a variety of effects on tumour progression.
-
Given the pleotropic nature of TGFβ signalling, the numerous downstream signalling events of TGFβ offer new and intriguing targets for therapeutic intervention.
-
Successful therapeutic targeting of TGFβ itself remains a highly desirable goal and the considerable advances in understanding TGFβ signalling, not only in the tumour epithelium but also in the tumour microenvironment, bring the realization of this goal closer.
Abstract
The influence of the microenvironment on tumour progression is becoming clearer. In this Review we address the role of an essential signalling pathway, that of transforming growth factor-β, in the regulation of components of the tumour microenvironment and how this contributes to tumour progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, P. M. & Massague, J. Cytostatic and apoptotic actions of TGFβ in homeostasis and cancer. Nature Rev. Cancer 3, 807–821 (2003).
Massague, J. G1 cell-cycle control and cancer. Nature 432, 298–306 (2004).
Gorsch, S. M., Memoli, V. A., Stukel, T. A., Gold, L. I. & Arrick, B. A. Immunohistochemical staining for transforming growth factor β 1 associates with disease progression in human breast cancer. Cancer Res. 52, 6949–6952 (1992).
Hasegawa, Y. et al. Transforming growth factor-β1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 91, 964–971 (2001).
Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Caulin, C., Scholl, F. G., Frontelo, P., Gamallo, C. & Quintanilla, M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-β 1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth Differ. 6, 1027–1035 (1995).
Walker, R. A., Dearing, S. J. & Gallacher, B. Relationship of transforming growth factor β 1 to extracellular matrix and stromal infiltrates in invasive breast carcinoma. Br. J. Cancer 69, 1160–1165 (1994).
Wikstrom, P., Stattin, P., Franck-Lissbrant, I., Damber, J. E. & Bergh, A. Transforming growth factor β1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 37, 19–29 (1998).
Hazelbag, S., Gorter, A., Kenter, G. G., van den Broek, L. & Fleuren, G. Transforming growth factor-β1 induces tumor stroma and reduces tumor infiltrate in cervical cancer. Hum. Pathol. 33, 1193–1199 (2002).
Bierie, B. & Moses, H. L. TGF-β and cancer. Cytokine Growth Factor Rev. 17, 29–40 (2006).
Massague, J. TGFβ signalling in context. Nature Rev. Mol. Cell Biol. 13, 616–630 (2012).
Rifkin, D. B. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J. Biol. Chem. 280, 7409–7412 (2005).
Shi, Y. & Massague, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Stenvers, K. L. et al. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol. Cell. Biol. 23, 4371–4385 (2003).
Moustakas, A. et al. The transforming growth factor β receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J. Biol. Chem. 268, 22215–22218 (1993).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Feng, X. H. & Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Schmierer, B. & Hill, C. S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor β-dependent nuclear accumulation of Smads. Mol. Cell. Biol. 25, 9845–9858 (2005).
Moustakas, A. & Heldin, C. H. Non-Smad TGF-β signals. J. Cell Sci. 118, 3573–3584 (2005).
Massague, J. & Gomis, R. R. The logic of TGFβ signaling. FEBS Lett. 580, 2811–2820 (2006).
Ewen, M. E., Oliver, C. J., Sluss, H. K., Miller, S. J. & Peeper, D. S. p53-dependent repression of CDK4 translation in TGF-β-induced G1 cell-cycle arrest. Genes Dev. 9, 204–217 (1995).
Polyak, K. et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 8, 9–22 (1994).
Hannon, G. J. & Beach, D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature 371, 257–261 (1994).
Pardali, K. & Moustakas, A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta 1775, 21–62 (2007).
Bottinger, E. P. et al. Expression of a dominant-negative mutant TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 16, 2621–2633 (1997).
Amendt, C., Schirmacher, P., Weber, H. & Blessing, M. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17, 25–34 (1998).
Huber, M. A., Kraut, N. & Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Dumont, N., Bakin, A. V. & Arteaga, C. L. Autocrine transforming growth factor-β signaling mediates Smad-independent motility in human cancer cells. J. Biol. Chem. 278, 3275–3285 (2003).
Giampieri, S. et al. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nature Cell Biol. 11, 1287–1296 (2009). This paper shows the identification of TGFβ signalling as a mediator of single-cell invasion and of vascular metastasis, with the cells that are unable to respond to TGFβ showing a cohesive migratory phenotype.
Muraoka-Cook, R. S. et al. Activated type I TGFβ receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 25, 3408–3423 (2006).
Safina, A., Vandette, E. & Bakin, A. V. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene 26, 2407–2422 (2007).
Schniewind, B. et al. Dissecting the role of TGF-β type I receptor/ALK5 in pancreatic ductal adenocarcinoma: Smad activation is crucial for both the tumor suppressive and prometastatic function. Oncogene 26, 4850–4862 (2007).
Esquela-Kerscher, A. & Slack, F. J. Oncomirs — microRNAs with a role in cancer. Nature Rev. Cancer 6, 259–269 (2006).
Yang, P. et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 22, 291–303 (2012).
Davis, B. N., Hilyard, A. C., Lagna, G. & Hata, A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61 (2008). This is the first established link between SMAD activity and the maturation of pri-miRNA through DROSHA, specifically following BMP treatment.
Wang, J., Wang, Y., Ma, Y., Lan, Y. & Yang, X. Transforming growth factor β-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J. Biol. Chem. 288, 10418–10426 (2013).
Liu, Y. et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 188, 5500–5510 (2012).
Wang, S. E. et al. Transforming growth factor β engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol. Cell. Biol. 28, 5605–5620 (2008).
Sartor, M. A. et al. ConceptGen: a gene set enrichment and gene set relation mapping tool. Bioinformatics 26, 456–463 (2010).
Hills, C. E., Willars, G. B. & Brunskill, N. J. Proinsulin C-peptide antagonizes the profibrotic effects of TGF-β1 via up-regulation of retinoic acid and HGF-related signaling pathways. Mol. Endocrinol. 24, 822–831 (2010).
Maupin, K. A. et al. Glycogene expression alterations associated with pancreatic cancer epithelial–mesenchymal transition in complementary model systems. PLoS ONE 5, e13002 (2010).
Coulouarn, C., Factor, V. M. & Thorgeirsson, S. S. Transforming growth factor-β gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology 47, 2059–2067 (2008).
Massague, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Mima, K. et al. Epithelial–mesenchymal transition expression profiles as a prognostic factor for disease-free survival in hepatocellular carcinoma: clinical significance of transforming growth factor-β signaling. Oncol. Lett. 5, 149–154 (2013).
Forrester, E. et al. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 65, 2296–2302 (2005).
Lu, S. L. et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 20, 1331–1342 (2006).
Paiva, C. E. et al. Absence of TGF-βRII predicts bone and lung metastasis and is associated with poor prognosis in stage III breast tumors. Cancer Biomark 11, 209–217 (2012). This paper is a histochemical study of TGFβ signalling in breast cancer and shows a correlation between decreased signalling in the tumour epithelium and increased tumour metastasis to the lungs and the bone. This article also indicates that low TGFBR2 levels indicate poor disease-free and overall survival.
Levy, L. & Hill, C. S. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 17, 41–58 (2006).
Malkoski, S. P. et al. Loss of transforming growth factor β type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin. Cancer Res. 18, 2173–2183 (2012).
Bierie, B. & Moses, H. L. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Rev. Cancer 6, 506–520 (2006).
Yang, L. et al. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 (2008).
Ijichi, H. et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J. Clin. Invest. 121, 4106–4117 (2011).
Lin, S. et al. Attenuation of TGF-β signaling suppresses premature senescence in a p21-dependent manner and promotes oncogenic Ras-mediated metastatic transformation in human mammary epithelial cells. Mol. Biol. Cell 23, 1569–1581 (2012).
Gewin, L. et al. TGF-β receptor deletion in the renal collecting system exacerbates fibrosis. J. Am. Soc. Nephrol. 21, 1334–1343 (2010).
Bierie, B. et al. Abrogation of TGF-β signaling enhances chemokine production and correlates with prognosis in human breast cancer. J. Clin. Invest. 119, 1571–1582 (2009). This paper is the first to establish of a poor clinical outcome related to the genetic changes, particularly the altered chemokine expression, that occur following ablation of TGFβ signalling in the tumour epithelium.
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature Med. 14, 518–527 (2008). This is the first identification of a link between stromal gene expression changes and the outcome of patients with breast cancer.
Knudsen, E. S. et al. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res. Treat. 133, 1009–1024 (2012).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Nombela-Arrieta, C., Ritz, J. & Silberstein, L. E. The elusive nature and function of mesenchymal stem cells. Nature Rev. Mol. Cell Biol. 12, 126–131 (2011).
Watabe, T. & Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 19, 103–115 (2009).
Cuiffo, B. G. & Karnoub, A. E. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh. Migr. 6, 220–230 (2012).
Tang, Y. et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Med. 15, 757–765 (2009).
Zhao, L. & Hantash, B. M. TGF-β1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam. Horm. 87, 127–141 (2011).
Kurpinski, K. et al. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28, 734–742 (2010).
Wan, M. et al. Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 30, 2498–2511 (2012).
Jung, Y. et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Commun. 4, 1795 (2013).
Quante, M. et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19, 257–272 (2011).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Spaeth, E. L. et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 4, e4992 (2009).
Shangguan, L. et al. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 30, 2810–2819 (2012).
Verona, E. V. et al. Transforming growth factor-β signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 67, 5737–5746 (2007).
Border, W. A. & Noble, N. A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 (1994).
Leask, A. & Abraham, D. J. TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 (2004).
Boyd, N. F. et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 356, 227–236 (2007).
Desmouliere, A., Geinoz, A., Gabbiani, F. & Gabbiani, G. Transforming growth factor-β 1 induces αalpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103–111 (1993).
Sime, P. J., Xing, Z., Graham, F. L., Csaky, K. G. & Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 100, 768–776 (1997).
Sonnylal, S. et al. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 56, 334–344 (2007).
Navab, R. et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc. Natl Acad. Sci. USA 108, 7160–7165 (2011).
Hawinkels, L. J. et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene http://dx.doi.org/10.1038/onc.2012.536 (2012).
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012). This paper establishes TGFβ-responsive stromal signalling, especially from fibroblasts, as a driver of tumour progression and metastasis. This stromal programme could be blocked with the addition of TGFβ inhibitors to slow tumour growth.
Bhowmick, N. A. et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004). This is the first report showing that genetic mutations in stromal fibroblast can result in the development of carcinoma formation from the adjacent epithelium.
Cheng, N. et al. Loss of TGF-β type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene 24, 5053–5068 (2005).
Franco, O. E. et al. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 71, 1272–1281 (2011).
Meng, W. et al. Downregulation of TGF-β receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer 11, 88 (2011).
Xu, B. J. et al. Quantitative analysis of the secretome of TGF-β signaling-deficient mammary fibroblasts. Proteomics 10, 2458–2470 (2010).
Bacman, D. et al. TGF-β receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-β1 expression in colon carcinoma: a retrospective study. BMC Cancer 7, 156 (2007).
Achyut, B. R. et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-β signaling. PLoS Genet. 9, e1003251 (2013). This is an interesting paper showing that inflammatory infiltrates mediate the pro-tumorigenic functions of fibroblasts that lack TGFβ signalling, particularly through the induction of epigenetic silencing of p15 and p16 in epithelial cells.
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H. & Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFβ. Nature Rev. Immunol. 10, 554–567 (2010).
Gigante, M., Gesualdo, L. & Ranieri, E. TGF-β: a master switch in tumor immunity. Curr. Pharm. Des. 18, 4126–4134 (2012).
Gong, D. et al. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 13, 31 (2012).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nature Rev. Immunol. 11, 723–737 (2011).
Fridlender, Z. G. & Albelda, S. M. Tumor-associated neutrophils: friend or foe? Carcinogenesis 33, 949–955 (2012).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009). This is the first paper to establish an N2 neutrophil phenotype and to show that this phenotype is driven by TGFβ signalling. These neutrophils promote tumour progression through the inhibition of antitumorigenic CD8+ T cell activity.
Marcoe, J. P. et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nature Immunol. 13, 843–850 (2012). This paper identifies TGFβ as a factor that inhibits NK cell maturation and shows that loss of TGFβ signalling is a mark of stage F NK cell development.
Leavy, O. Maturation and function of NK cells. Nature Rev. Immunol. 12, 150 (2012).
Laouar, Y., Sutterwala, F. S., Gorelik, L. & Flavell, R. A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nature Immunol. 6, 600–607 (2005).
Tanaka, H. et al. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol. Rep. 24, 1637–1643 (2010).
Novitskiy, S. V. et al. Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties. J. Leukoc. Biol. 92, 641–651 (2012).
Perrot, I. et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 178, 2763–2769 (2007).
Roncarolo, M. G., Levings, M. K. & Traversari, C. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 193, F5–9 (2001).
Shull, M. M. et al. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 (1992).
Kulkarni, A. B. et al. Transforming growth factor β 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA 90, 770–774 (1993).
Marie, J. C., Liggitt, D. & Rudensky, A. Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25, 441–454 (2006).
Bommireddy, R. et al. Self-antigen recognition by TGF β1-deficient T cells causes their activation and systemic inflammation. Lab Invest. 86, 1008–1019 (2006).
Li, M. O. & Flavell, R. A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunol. 9, 194–202 (2008).
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nature Rev. Immunol. 8, 523–532 (2008).
von Boehmer, H. & Daniel, C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nature Rev. Drug Discov. 12, 51–63 (2013).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006). This paper identifies TGFβ as a primary factor in the induced differentiation of T H 17 cell commitment in a subpopulation of T cells — a process that is independent of T Reg cells.
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Whiteside, T. L. What are regulatory T cells (Treg) regulating in cancer and why? Semin. Cancer Biol. 22, 327–334 (2012).
Kim, B. G. et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441, 1015–1019 (2006). This seminal paper identifies of TGFβ superfamily signalling in T cells as an inhibitor of tumour progression due to the suppression of the expression of T H 1 cytokines.
Ghoreschi, K. et al. Generation of pathogenic Th17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Jain, R. K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Liu, J. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012). This is an interesting paper showing that TGFβ inhibition-normalized breast tumour stroma enhances chemotherapeutic efficacy.
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y. & Jain, R. K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Mead, A. L., Wong, T. T., Cordeiro, M. F., Anderson, I. K. & Khaw, P. T. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest. Ophthalmol. Vis. Sci. 44, 3394–3401 (2003).
Bogdahn, U. et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 13, 132–142 (2011).
Schlingensiepen, K. H. et al. Transforming growth factor-β 2 gene silencing with trabedersen in pancreatic cancer. Cancer Sci. 102, 1193–1200 (2011).
Roldan Urgoiti, G. B., Singh, A. D. & Easaw, J. C. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J. Neurooncol. 108, 173–177 (2012).
Akhurst, R. J. & Hata, A. Targeting the TGFβ signalling pathway in disease. Nature Rev. Drug Discov. 11, 790–811 (2012).
Zhong, Z. et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin. Cancer Res. 16, 1191–1205 (2010). This paper shows that anti-TGFβ therapeutics slow tumour progression by modulating the composition of immune cell infiltrates in favour of an antitumorigenic phenotype.
Uhl, M. et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 64, 7954–7961 (2004).
Kim, S. et al. Systemic blockade of transforming growth factor-β signaling augments the efficacy of immunogene therapy. Cancer Res. 68, 10247–10256 (2008).
Foster, A. E. et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J. Immunother. 31, 500–505 (2008).
Azad, N., Zahnow, C. A., Rudin, C. M. & Baylin, S. B. The future of epigenetic therapy in solid tumours-lessons from the past. Nature Rev. Clin. Oncol. 10, 256–266 (2013).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nature Rev. Genet. 3, 415–428 (2002).
Zhang, Q. et al. TGF-β regulates DNA methyltransferase expression in prostate cancer, correlates with aggressive capabilities, and predicts disease recurrence. PLoS ONE 6, e25168 (2011).
Ammanamanchi, S. & Brattain, M. G. Restoration of transforming growth factor-β signaling through receptor RI induction by histone deacetylase activity inhibition in breast cancer cells. J. Biol. Chem. 279, 32620–32625 (2004).
Kang, S. H. et al. Transcriptional repression of the transforming growth factor-β type I receptor gene by DNA methylation results in the development of TGF-β resistance in human gastric cancer. Oncogene 18, 7280–7286 (1999).
Osada, H. et al. Heterogeneous transforming growth factor (TGF)-β unresponsiveness and loss of TGF-β receptor type II expression caused by histone deacetylation in lung cancer cell lines. Cancer Res. 61, 8331–8339 (2001).
Khin, S. S. et al. Epigenetic alteration by DNA promoter hypermethylation of genes related to transforming growth factor-β (TGF-B) signaling in cancer. Cancers 3, 982–993 (2011).
Yeh, K. T. et al. Aberrant TGFβ/SMAD4 signaling contributes to epigenetic silencing of a putative tumor suppressor, RunX1T1 in ovarian cancer. Epigenetics 6, 727–739 (2011).
Papageorgis, P. et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 70, 968–978 (2010).
Pan, X., Chen, Z., Huang, R., Yao, Y. & Ma, G. Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS ONE 8, e60335 (2013).
Hu, B., Gharaee-Kermani, M., Wu, Z. & Phan, S. H. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am. J. Pathol. 177, 21–28 (2010).
Ross, S. et al. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 25, 4490–4502 (2006).
Sun, G. et al. Epigenetic histone methylation modulates fibrotic gene expression. J. Am. Soc. Nephrol. 21, 2069–2080 (2010).
Glenisson, W., Castronovo, V. & Waltregny, D. Histone deacetylase 4 is required for TGFβ1-induced myofibroblastic differentiation. Biochim. Biophys. Acta 1773, 1572–1582 (2007).
Diaz-Valdes, N. et al. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFβ1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Res. 71, 812–821 (2011).
Terabe, M. et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin. Cancer Res. 15, 6560–6569 (2009).
Hardee, M. E. et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 72, 4119–4129 (2012).
Noma, K. et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology 134, 1981–1993 (2008).
Mazzocca, A., Fransvea, E., Lavezzari, G., Antonaci, S. & Giannelli, G. Inhibition of transforming growth factor β receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology 50, 1140–1151 (2009).
Zhang, M. et al. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia 13, 537–549 (2011).
Tran, T. T. et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 9, 259–270 (2007).
Garrison, K. et al. The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol. Immunother. 61, 511–521 (2012).
Lucas, P. J., Kim, S. J., Melby, S. J. & Gress, R. E. Disruption of T. cell homeostasis in mice expressing a T. cell-specific dominant negative transforming growth factor β II receptor. J. Exp. Med. 191, 1187–1196 (2000).
Acknowledgements
This work is supported by US National Institutes of Health (NIH) Grants CA085492 and CA102162. The authors would also like to thank all of the members of the Moses Laboratory for their thoughtful discussions. In addition, the authors would like to apologize to any investigators whose work could not be included owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Epithelial-to-mesenchymal transition
-
The process by which epithelial cells lose their epithelial characteristics, such as cellular polarity and cell–cell junctions, in favour of mesenchymal characteristics, which include high cellular motility.
- Cohesive migratory phenotype
-
Contrary to single-cell invasion, this is a form of invasion by which cells maintain their cell–cell contacts and by which cells migrate as a unit.
- MicroRNA
-
(miRNA). 20–30 nucleotide long non-coding RNA that modulates gene expression by binding to complementary sequences in the 3′ untranslated region of target genes, which induces repression of gene translation or mRNA degradation.
- Myeloid-derived suppressor cells
-
(MDSCs). A heterogeneous population of cells that are defined by their myeloid origin, immature state and ability to potently suppress T cell responses.
- Chemotaxis
-
The function of a cell responding to a chemokine gradient to promote directional migration towards sites of tissue injury or into tumour microenvironments.
- Dominant-negative
-
A defective protein product that effectively inhibits the function of its wild-type counterpart by retaining essential interaction capabilities without having a desired effector function.
- Desmoplasia
-
The accumulation of extracellular matrix proteins, usually through the enhanced activation of stromal fibroblasts.
- Vogelgram
-
A model for colorectal cancer development in which specific genetic changes are acquired, which mark progression of the disease from benign to malignant.
- Myofibroblast
-
A generalized term for an activated fibroblast. In tumour biology, these cells function to deposit and to remodel the extracellular matrix as well as to alter tumour progression through paracrine protein signalling.
- T helper 1 cell
-
(TH1 cell). A primary effector cell of the adaptive immune system, which is classically defined as antitumorigenic.
- 1D11
-
An antibody which is specifically directed towards the three transforming growth factor-β ligands and used to sequester these ligands, thus inhibiting TGFβ signalling.
- Losartan
-
An angiotensin II receptor antagonist that has been shown to have the off-target effects of downregulating expression of type I transforming growth factor-β receptor (Tgfbr1) and Tgfbr2.
Rights and permissions
About this article
Cite this article
Pickup, M., Novitskiy, S. & Moses, H. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13, 788–799 (2013). https://doi.org/10.1038/nrc3603
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3603
This article is cited by
-
Dual effect of vitamin D3 on breast cancer-associated fibroblasts
BMC Cancer (2024)
-
Deciphering the role of neddylation in tumor microenvironment modulation: common outcome of multiple signaling pathways
Biomarker Research (2024)
-
Nasopharyngeal carcinoma: current views on the tumor microenvironment's impact on drug resistance and clinical outcomes
Molecular Cancer (2024)
-
Multi-omic analyses of m5C readers reveal their characteristics and immunotherapeutic proficiency
Scientific Reports (2024)
-
Neutrophils in cancer: dual roles through intercellular interactions
Oncogene (2024)