Key Points
-
Warburg observed, 70 years ago, that tumours produce excess lactate in the presence of oxygen. This became known as aerobic glycolysis or the 'Warburg effect' which he interpreted as mitochondrial dysfunction. However, it is now clear that mitochondrial function is essential for cancer cell viability, because elimination of cancer cell mitochondrial DNAs (mtDNAs) reduces their growth rate and tumorigenicity.
-
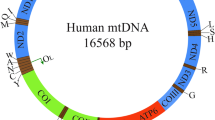
The mitochondrial genome encompasses thousands of copies of the mtDNA and more than one thousand nuclear DNA (nDNA)-encoded genes. mtDNA mutations have been found in various cancers and seem to alter mitochondrial metabolism, enhance tumorigenesis and permit cancer cell adaptation to changing environments.
-
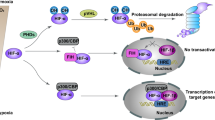
Mutations in nDNA genes involved in mitochondrial metabolism, including succinate dehydrogenase (SDH), fumarate hydratase (FH), isocitrate dehydrogenase 1 (IDH1) and IDH2, result in increased succinate, fumarate, or R(-)-2-hydroxyglutarate levels. These metabolic alterations can inhibit various α-ketoglutarate-dependent dioxygenases; they can also activate the NFE2-related factor 2 (NRF2) stress response pathway. All of these effects can contribute to tumorigenesis.
-
Activation of signalling pathways and oncogenes that are known to be important in tumorigenesis also affect mitochondrial function. The PI3K–PTEN–AKT pathway shifts metabolism from oxidative to glycolytic, thus permitting the redistribution of glycolytic nutrients from catabolism to anabolism. Activation of MYC induces glutaminolysis, which provides anaplerotic substrates to the mitochondrial tricarboxylic acid cycle, thus enhancing citrate production and its export to the cytosol to provide acetyl-CoA for lipid biogenesis and protein modifications.
-
Altered mitochondrial metabolism can increase the production of mitochondrial reactive oxygen species (ROS) and change the cellular redox status, thus altering the activities of transcription factors such as HIF1α and FOS–JUN to change gene expression and stimulate cancer cell proliferation.
-
A decrease of the mitochondrial membrane potential or mutation of the promyelocytic leukaemia (PML) gene reduces mitochondrial Ca2+ uptake, thus decreasing the activation of the mitochondrial intrinsic apoptosis pathway.
-
Reduced mitochondrial Ca2+ retention increases the cytosolic Ca2+ concentration. This activates mitochondrial retrograde signalling through stimulation of calcineurin and IκBβ-dependent NF-κB, activation of enhanceosome-driven transcription and increased metastatic potential.
-
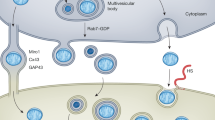
Cancer cell ROS production inactivates caveolin 1 in adjacent stromal fibroblasts. This increases mitophagy, reduces mitochondrial function and increases lactate production in these fibroblasts. Secreted stromal cell lactate then fuels cancer cell oxidative metabolism, which drives tumour growth and proliferation. This is known as the 'reverse Warburg effect'.
Abstract
Contrary to conventional wisdom, functional mitochondria are essential for the cancer cell. Although mutations in mitochondrial genes are common in cancer cells, they do not inactivate mitochondrial energy metabolism but rather alter the mitochondrial bioenergetic and biosynthetic state. These states communicate with the nucleus through mitochondrial 'retrograde signalling' to modulate signal transduction pathways, transcriptional circuits and chromatin structure to meet the perceived mitochondrial and nuclear requirements of the cancer cell. Cancer cells then reprogramme adjacent stromal cells to optimize the cancer cell environment. These alterations activate out-of-context programmes that are important in development, stress response, wound healing and nutritional status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Warburg, O. The Metabolism of Tumors (R. R. Smith, 1931).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Wallace, D. C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 70, 363–374 (2005).
Lin, M. Molecular imaging using positron emission tomography in colorectal cancer. Discov. Med. 11, 435–447 (2011).
Pedersen, P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog . Exp. Tumor Res. 22, 190–274 (1978).
Lane, N. & Martin, W. The energetics of genome complexity. Nature 467, 929–934 (2010).
Wallace, D. C. Why do we have a maternally inherited mitochondrial DNA?Insights from evolutionary medicine. Annu. Rev. Biochem. 76, 781–821 (2007).
Horton, T. M. et al. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes Chromosomes Cancer 15, 95–101 (1996).
Desjardins, P., Frost, E. & Morais, R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol. Cell. Biol. 5, 1163–1169 (1985).
Desjardins, P., de Muys, J. M. & Morais, R. An established avian fibroblast cell line without mitochondrial DNA. Somat. Cell Genet. 12, 133–139 (1986).
King, M. P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
Magda, D. et al. mtDNA depletion confers specific gene expression profiles in human cells grown in culture and in xenograft. BMC Genomics 9, 521 (2008).
Morais, R. et al. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Res. 54, 3889–3896 (1994).
Cavalli, L. R., Varella-Garcia, M. & Liang, B. C. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 8, 1189–1198 (1997).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Murgia, M., Giorgi, C., Pinton, P. & Rizzuto, R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J. Mol. Cell. Cardiol. 46, 781–788 (2009).
Aanen, D. K. & Maas, M. F. Recruitment of healthy mitochondria fuels transmissible cancers. Trends Genet. 28, 1–6 (2012).
Rebbeck, C. A., Leroi, A. M. & Burt, A. Mitochondrial capture by a transmissible cancer. Science 331, 303 (2011).
Brandon, M., Baldi, P. & Wallace, D. C. Mitochondrial mutations in cancer. Oncogene 25, 4647–4662 (2006).
Chinnery, P. F., Samuels, D. C., Elson, J. & Turnbull, D. M. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet 360, 1323–1325 (2002).
Copeland, W. C., Wachsman, J. T., Johnson, F. M. & Penta, J. S. Mitochondrial DNA alterations in cancer. Cancer Invest. 20, 557–569 (2002).
Gasparre, G. et al. Clonal expansion of mutated mitochondrial DNA is associated with tumor formation and complex I deficiency in the benign renal oncocytoma. Hum. Mol. Genet. 17, 986–995 (2008).
Bartoletti-Stella, A. et al. Mitochondrial DNA mutations in oncocytic adnexal lacrimal glands of the conjunctiva. Arch. Ophthalmol. 129, 664–666 (2011).
Pereira, L., Soares, P., Maximo, V. & Samuels, D. C. Somatic mitochondrial DNA mutations in cancer escape purifying selection and high pathogenicity mutations lead to the oncocytic phenotype: pathogenicity analysis of reported somatic mtDNA mutations in tumors. BMC Cancer 12, 53 (2012).
Salas, A. et al. A critical reassessment of the role of mitochondria in tumorigenesis. PLoS Med. 2, e296 (2005).
Meierhofer, D. et al. Mitochondrial DNA mutations in renal cell carcinomas revealed no general impact on energy metabolism. Br. J. Cancer 94, 268–274 (2006).
Czarnecka, A. M. et al. Molecular oncology focus - is carcinogenesis a 'mitochondriopathy'? J. Biomed. Sci. 17, 31 (2010).
Howell, A. N. & Sager, R. Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc. Natl Acad. Sci. USA 75, 2358–2362 (1978).
Petros, J. A. et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl Acad. Sci. USA 102, 719–724 (2005). A demonstration that human mtDNA mutations that increase ROS production enhance tumorigenesis, whereas normal mtDNAs suppress tumorigenesis.
Shidara, Y. et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 65, 1655–1663 (2005).
Ishikawa, K. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008). This study shows that mouse mtDNA mutations that increase mitochondrial ROS levels also increase tumorigenesis.
Canter, J. A., Kallianpur, A. R., Parl, F. F. & Millikan, R. C. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 65, 8028–8033 (2005).
Liu, V. W. et al. Mitochondrial DNA variant 16189T>C is associated with susceptibility to endometrial cancer. Hum. Mut. 22, 173–174 (2003).
Permuth-Wey, J. et al. Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev. 20, 1131–1145 (2011).
Zhang, J. et al. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc. Natl Acad. Sci. USA 100, 1116–1121 (2003).
Zhai, K., Chang, L., Zhang, Q., Liu, B. & Wu, Y. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion 11, 559–563 (2011).
Wallace, D. C. Bioenergetic origins of complexity and diseases. Cold Spring Harb. Symp. Quant. Biol. 76, 1–16 (2011).
Ruiz-Pesini, E. & Wallace, D. C. Evidence for adaptive selection acting on the tRNA and rRNA genes of the human mitochondrial DNA. Hum. Mut. 27, 1072–1081 (2006).
Mishmar, D. et al. Natural selection shaped regional mtDNA variation in humans. Proc. Natl Acad. Sci. USA 100, 171–176 (2003).
Wallace, D. C. Colloquium paper: bioenergetics, the origins of complexity, and the ascent of man. Proc. Natl Acad. Sci. USA 107, 8947–8953 (2010).
Parrella, P. et al. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 61, 7623–7626 (2001).
Guo, J. et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 71, 2978–2987 (2011).
Han, B. et al. Human mitochondrial transcription factor A functions in both nuclei and mitochondria and regulates cancer cell growth. Biochem. Biophys. Res. Commun. 408, 45–51 (2011).
Bogenhagen, D. F., Rousseau, D. & Burke, S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 283, 3665–3675 (2008).
Bogenhagen, D. F. Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta 1819, 914–920 (2011).
Khidr, L. et al. Role of SUV3 helicase in maintaining mitochondrial homeostasis in human cells. J. Biol. Chem. 283, 27064–27073 (2008).
Chen, P.-L. et al. Mitochondrial genome instability resulting from SUV3 haploinsufficiency leads to tumorigenesis and shortened lifespan. Oncogene 7 May 2012 (doi:10.1038/onc.2012.120).
Bardella, C., Pollard, P. J. & Tomlinson, I. SDH mutations in cancer. Biochim. Biophys. Acta 1807, 1432–1443 (2011).
Baysal, B. E. et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000). The first report that inactivation of SDHD can cause paragangliosis.
Niemann, S. & Muller, U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nature Genet. 26, 268–270 (2000).
Astuti, D. et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet. 69, 49–54 (2001).
Kurelac, I., Romeo, G. & Gasparre, G. Mitochondrial metabolism and cancer. Mitochondrion 11, 635–637 (2011).
Wallace, D. C. & Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 10, 12–31 (2010).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000). Evidence that increased mitochondrial ROS levels can inactivate PHDs and activate HIF1α.
Guzy, R. D., Sharma, B., Bell, E., Chandel, N. S. & Schumacker, P. T. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biochem. 28, 718–731 (2008).
Selak, M. A., Duran, R. V. & Gottlieb, E. Redox stress is not essential for the pseudo-hypoxic phenotype of succinate dehydrogenase deficient cells. Biochim. Biophys. Acta 1757, 567–572 (2006).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012). A demonstration that excessive dicarboxylic acids from SDH and FH inactivation inhibit α-ketoglutarate-dependent dioxygenases, thus altering chromatin structure and gene expression.
Picaud, S. et al. Structural basis of fumarate hydratase deficiency. J. Inherit. Metab. Dis. 34, 671–676 (2011).
Frezza, C. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 477, 225–228 (2011). A report that increased fumarate levels activates the NRF2 stress response, inducing HMOX1 and haem degradation.
Adam, J. et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20, 524–537 (2011).
Stark, R. et al. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J. Biol. Chem. 284, 26578–26590 (2009).
Kibbey, R. G. et al. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 5, 253–264 (2007).
Thompson, C. B. Metabolic enzymes as oncogenes or tumor suppressors. New Engl. J. Med. 360, 813–815 (2009).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Ward, P. S. et al. Identification of additional IDH mutations associated with oncometabolite R(–)-2-hydroxyglutarate production. Oncogene 31, 2491–2498 (2012).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009). This study reports that heterozygous IDH1 mutation creates a neomorphic enzyme that generates the novel metabolite ( R )-2HG.
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012). This study shows that in contrast to succinate and fumarate, ( R )-2HG does not inactivate PHDs and activate HIF1α, implying that tumorigenesis involves an alternative pathway.
Figueroa, M. E. et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 17, 13–27 (2010).
Chowdhury, R. et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 12, 463–469 (2011).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–483 (2012).
Yoon, J. C. et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 24, 1507–1518 (2010).
Bjornsson, H. T. et al. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J. Natl Cancer Inst. 99, 1270–1273 (2007).
Feinberg, A. P. Phenotypic plasticity and the epigenetics of human disease. Nature 447, 433–440 (2007).
Feinberg, A. P. Epigenetics at the epicenter of modern medicine. JAMA 299, 1345–1350 (2008).
Smiraglia, D. J., Kulawiec, M., Bistulfi, G. L., Gupta, S. G. & Singh, K. K. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol. Ther. 7, 1182–1190 (2008).
Naviaux, R. K. Mitochondrial control of epigenetics. Cancer Biol. Ther. 7, 1191–1193 (2008).
Toye, A. A. et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 48, 675–686 (2005).
Freeman, H. C., Hugill, A., Dear, N. T., Ashcroft, F. M. & Cox, R. D. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55, 2153–2156 (2006).
Collins, S., Martin, T. L., Surwit, R. S. & Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol. Behav. 81, 243–248 (2004).
Nicholson, A. et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity 18, 1902–1905 (2010).
Huang, T. T. et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum. Mol. Genet. 15, 1187–1194 (2006).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012). This study shows that mitochondrial α-ketoglutarate can be reductively carboxylated using mitochondrial NADPH to increase citrate production.
Wallace, D. C., Fan, W. & Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 5, 297–348 (2010).
Melov, S. et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl Acad. Sci. USA 96, 846–851 (1999).
Yan, L. J., Levine, R. L. & Sohal, R. S. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl Acad. Sci. USA 94, 11168–11172 (1997).
Tong, J., Schriner, S. E., McCleary, D., Day, B. J. & Wallace, D. C. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for Neurofibromatosis-1 in Drosophila melanogaster. Nature Genet. 39, 476–485 (2007).
Sasaki, M. et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488, 656–659 (2012).
Gupta, S. C. et al. Upsides and downsides of ROS for cancer: the roles of ROS in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 16, 1295–1322 (2012).
Burdon, R. H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Rad. Biol. Med. 18, 775–794 (1995).
Lander, H. M. An essential role for free radicals and derived species in signal transduction. FASEB J. 11, 118–124 (1997).
Abate, C., Patel, L., Rauscher, F. J. & Curran, T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249, 1157–1161 (1990). The first demonstration that FOS and JUN are regulated by cysteine oxidation–reduction.
Liu, H., Colavitti, R., Rovira, I. I. & Finkel, T. Redox-dependent transcriptional regulation. Circul. Res. 97, 967–974 (2005).
Ordway, J. M., Eberhart, D. & Curran, T. Cysteine 64 of Ref-1 is not essential for redox regulation of AP-1 DNA binding. Mol. Cell. Biol. 23, 4257–4266 (2003).
Xanthoudakis, S., Miao, G. G. & Curran, T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc. Natl Acad. Sci. USA 91, 23–27 (1994).
Go, Y. M. & Jones, D. P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 1780, 1273–1290 (2008).
Jones, D. P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 295, C849–C868 (2008).
Kemp, M., Go, Y. M. & Jones, D. P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Rad. Biol. Med. 44, 921–937 (2008).
Guzy, R. D., Mack, M. M. & Schumacker, P. T. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid. Redox Signal. 9, 1317–1328 (2007).
Sanchez-Cenizo, L. et al. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J. Biol. Chem. 285, 25308–25313 (2010).
Willers, I. M. & Cuezva, J. M. Post-transcriptional regulation of the mitochondrial H+-ATP synthase: a key regulator of the metabolic phenotype in cancer. Biochim. Biophys. Acta 1807, 543–551 (2011).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 23, 537–548 (2009).
Wise, D. R. & Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 (2010).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008). A report that activation of the PI3K–PTEN–AKT pathway redirects cellular metabolism from oxidative catabolism to glycolytic anabolism, thus enhancing cancer cell biogenesis.
Pedersen, P. L., Mathupala, S., Rempel, A., Geschwind, J. F. & Ko, Y. H. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim. Biophys. Acta 1555, 14–20 (2002).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004).
Nemoto, S. & Finkel, T. Ageing and the mystery at Arles. Nature 429, 149–152 (2004).
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005). An overview of mitochondrial biology and genetics and their relation to disease.
Daitoku, H., Yamagata, K., Matsuzaki, H., Hatta, M. & Fukamizu, A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52, 642–649 (2003).
Spiegelman, B. M. & Heinrich, R. Biological control through regulated transcriptional coactivators. Cell 119, 157–167 (2004).
Ferber, E. C. et al. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 19, 968–979 (2012).
Li, F. et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 25, 6225–6234 (2005).
Bluemlein, K. et al. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget 2, 393–400 (2011).
Hitosugi, T. et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2, ra73 (2009).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
Gruning, N. M. et al. Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 14, 415–427 (2011). A demonstration that oxidation of PKM2 inhibits glycolysis and redirects substrates into the pentose phosphate pathway to synthesize NADPH and enhance antioxidant defences.
Hamanaka, R. B. & Chandel, N. S. Warburg effect and redox balance. Science 334, 1219–1220 (2011).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 (2012).
Hoyos, B., Acin-Perez, R., Fischman, D. A., Manfredi, G. & Hammerling, U. Hiding in plain sight: uncovering a new function of vitamin A in redox signaling. Biochim. Biophys. Acta 1821, 241–247 (2012).
Zaugg, K. et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25, 1041–1051 (2011).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007). This study shows that MYC induction of glutaminolysis provides anaplerotic TCA cycle intermediates to generate citrate and sustain cytosolic fatty acid synthesis.
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008).
Bensaad, K., Cheung, E. C. & Vousden, K. H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 28, 3015–3026 (2009).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Sahin, E. & Depinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010).
Fukuda, R. et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 (2007).
Semenza, G. L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 405, 1–9 (2007).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Zhang, H. et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903 (2008).
Semenza, G. L. Mitochondrial autophagy: life and breath of the cell. Autophagy 4, 534–536 (2008).
Devlin, C., Greco, S., Martelli, F. & Ivan, M. miR-210: more than a silent player in hypoxia. IUBMB Life 63, 94–100 (2011).
Bell, E. L., Emerling, B. M., Ricoult, S. J. & Guarente, L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30, 2986–2996 (2011).
Yang, J. et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. J. Clin. Invest. 122, 600–611 (2012).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Luo, W. & Semenza, G. L. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2, 551–556 (2011).
McCormack, J. G. & Denton, R. M. A comparative study of the regulation of Ca2+ of the activities of the 2-oxoglutarate dehydrogenase complex and NAD+-isocitrate dehydrogenase from a variety of sources. Biochem. J. 196, 619–624 (1981).
McCormack, J. G., Halestrap, A. P. & Denton, R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425 (1990).
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Pinton, P., Giorgi, C. & Pandolfi, P. P. The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ. 18, 1450–1456 (2011).
Martinez-Caballero, S. et al. Assembly of the mitochondrial apoptosis-induced channel, MAC. J. Biol. Chem. 284, 12235–12245 (2009).
Peixoto, P. M., Ryu, S. Y., Bombrun, A., Antonsson, B. & Kinnally, K. W. MAC inhibitors suppress mitochondrial apoptosis. Biochem. J. 423, 381–387 (2009).
Dejean, L. M. et al. MAC and Bcl-2 family proteins conspire in a deadly plot. Biochim. Biophys. Acta 1797, 1231–1238 (2010).
Amuthan, G. et al. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 20, 1910–1920 (2001). A demonstration that a decrease in cancer cell mtDNA content and membrane potential increases cytosolic Ca2+, activates 'retrograde signalling', and increases epithelial–mesenchymal transition and cellular invasiveness.
Biswas, G. et al. A distinctive physiological role for IκBβ in the propagation of mitochondrial respiratory stress signaling. J. Biol. Chem. 283, 12586–12594 (2008).
Guha, M., Srinivasan, S., Biswas, G. & Avadhani, N. G. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J. Biol. Chem. 282, 14536–14546 (2007).
Guha, M., Pan, H., Fang, J. K. & Avadhani, N. G. Heterogeneous nuclear ribonucleoprotein A2 is a common transcriptional coactivator in the nuclear transcription response to mitochondrial respiratory stress. Mol. Biol. Cell 20, 4107–4119 (2009).
Guha, M., Fang, J. K., Monks, R., Birnbaum, M. J. & Avadhani, N. G. Activation of Akt is essential for the propagation of mitochondrial respiratory stress signaling and activation of the transcriptional coactivator heterogeneous ribonucleoprotein A2. Mol. Biol. Cell 21, 3578–3589 (2010).
Guha, M., Tang, W., Sondheimer, N. & Avadhani, N. G. Role of calcineurin, hnRNPA2 and Akt in mitochondrial respiratory stress-mediated transcription activation of nuclear gene targets. Biochim. Biophys. Acta 1797, 1055–1065 (2010).
Fan, W., Lin, C. S., Potluri, P., Procaccio, V. & Wallace, D. C. MtDNA lineage analysis of mouse L cell lines reveals the accumulation of multiple mtDNA mutants and intermolecular recombination. Genes Dev. 26, 384–394 (2012).
Park, J. S. et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 18, 1578–1589 (2009).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Goh, J. et al. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer 11, 191 (2011).
Woo, D. K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APCMin./+ mice. Am. J. Pathol. 180, 24–31 (2012).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Zu, X. L. & Guppy, M. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 313, 459–465 (2004).
Bonuccelli, G. et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 9, 1960–1971 (2010).
Pavlides, S. et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer's disease, and “Neuron-Glia Metabolic Coupling”. Aging 2, 185–199 (2010).
Castello-Cros, R. et al. Matrix remodeling stimulates stromal autophagy, “fueling” cancer cell mitochondrial metabolism and metastasis. Cell Cycle 10, 2021–2034 (2011). This study shows that cancer cell ROS production inactivates stromal cell caveolin 1, thus inducing stromal lactate production that feeds cancer cell oxidative metabolism and growth, a process known as the 'reverse Warburg effect'.
Capparelli, C. et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle 11, 2285–2302 (2012).
Pavlides, S. et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid. Redox Signal. 16, 1264–1284 (2012).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Phillips, D. et al. Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1034–R1048 (2012).
Wallace, D. C. The epigenome and the mitochondrion: bioenergetics and the environment. Genes Dev. 24, 1571–1573 (2010).
Fan, W. et al. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science 319, 958–962 (2008).
Hashizume, O. et al. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc. Natl Acad. Sci. USA 109, 10528–10533 (2012).
Oliver, N. A. & Wallace, D. C. Assignment of two mitochondrially synthesized polypeptides to human mitochondrial DNA and their use in the study of intracellular mitochondrial interaction. Mol. Cell. Biol. 2, 30–41 (1982).
Acknowledgements
The author would like to thank L. Adang and M. Lott for their assistance in preparing this manuscript. This work was supported by the US National Institutes of Health (NIH) grants NS21328, NS070298, AG24373 and DK73691, and a Simons Foundation Grant 205844.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Wallace, D. Mitochondria and cancer. Nat Rev Cancer 12, 685–698 (2012). https://doi.org/10.1038/nrc3365
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3365
This article is cited by
-
Ovarian cancer cells regulate their mitochondrial content and high mitochondrial content is associated with a poor prognosis
BMC Cancer (2024)
-
Prognosis prediction and risk stratification of breast cancer patients based on a mitochondria-related gene signature
Scientific Reports (2024)
-
Artificial intelligence in pathological anatomy: digitization of the calculation of the proliferation index (Ki-67) in breast carcinoma
Artificial Life and Robotics (2024)
-
Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases
Neuroscience Bulletin (2024)
-
Expression of Candidate Gene NDUFS1 in Breast Cancer: An in-silico Approach
Indian Journal of Gynecologic Oncology (2024)