Key Points
-
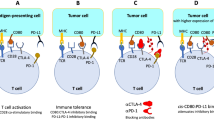
Photodynamic therapy (PDT) uses non-toxic dyes and harmless visible light in combination with oxygen to produce highly reactive oxygen species that kill cells.
-
In addition to destroying tumour tissue by a process that can produce cellular necrosis and the expression of stress proteins, PDT produces an acute inflammation, and attracts leukocytes to treated tumours.
-
PDT might increase the immunogenicity of dead tumour cells by exposing or creating new antigens, and by inducing heat-shock proteins that increase the efficiency of antigen cross-presentation to form more effective tumour-specific cytotoxic T cells.
-
The pro-inflammatory effects of PDT might increase dendritic-cell migration, antigen uptake and maturation.
-
PDT can produce tumour cures and long-lasting tumour-specific immunity (memory), as has been shown by the rejection of tumours on rechallenge in certain mouse and rat models.
-
PDT has been combined with a range of immunostimulatory therapies, including microbial adjuvants, to increase the anti-tumour immunity produced by PDT alone.
-
There are only a few reports of the immunostimulatory effects of PDT in humans, but increasing recognition of the effect should lead to further work and possibly to improved patient outcome.
Abstract
Photodynamic therapy (PDT) uses non-toxic photosensitizers and harmless visible light in combination with oxygen to produce cytotoxic reactive oxygen species that kill malignant cells by apoptosis and/or necrosis, shut down the tumour microvasculature and stimulate the host immune system. In contrast to surgery, radiotherapy and chemotherapy that are mostly immunosuppressive, PDT causes acute inflammation, expression of heat-shock proteins, invasion and infiltration of the tumour by leukocytes, and might increase the presentation of tumour-derived antigens to T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moan, J. & Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 23, 3591–3600 (2003).
Dolmans, D. E., Fukumura, D. & Jain, R. K. Photodynamic therapy for cancer. Nature Rev. Cancer 3, 380–387 (2003). A recent review on the theory and applications of PDT in cancer treatment.
Detty, M. R., Gibson, S. L. & Wagner, S. J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 47, 3897–3915 (2004).
Castano, A. P., Demidova, T. N. & Hamblin, M. R. Mechanisms in photodynamic therapy: part two — cellular signalling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2, 1–23 (2005).
Castano, A. P., Demidova, T. N. & Hamblin, M. R. Mechanisms in photodynamic therapy: part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2, 91–106 (2005).
Oleinick, N. L., Morris, R. L. & Belichenko, I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem. Photobiol. Sci. 1, 1–21 (2002).
Krammer, B. Vascular effects of photodynamic therapy. Anticancer Res. 21, 4271–4277 (2001).
Dolmans, D. E. et al. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 62, 2151–2156 (2002).
Korbelik, M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. Surg. 14, 329–334 (1996).
van Duijnhoven, F. H. et al. The immunological consequences of photodynamic treatment of cancer, a literature review. Immunobiology 207, 105–113 (2003).
Canti, G., De Simone, A. & Korbelik, M. Photodynamic therapy and the immune system in experimental oncology. Photochem. Photobiol. Sci. 1, 79–80 (2002).
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Friedman, E. J. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr. Pharm. Des. 8, 1765–1780 (2002).
Crum, E. D. Effect of cisplatin upon expression of in vivo immune tumor resistance. Cancer Immunol. Immunother. 36, 18–24 (1993).
Sierra-Rivera, E., Voorhees, G. J. & Freeman, M. L. g irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat. Res. 135, 40–45 (1993).
Ng, C. S. et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J. Invest. Surg. 18, 81–88 (2005).
Fontana, G. et al. Immunological response to prostatic cancer cryotherapy: certainties, controversies, hypotheses. Prog. Clin. Biol. Res. 303, 299–300 (1989).
Ivarsson, K. et al. Resistance to tumour challenge after tumour laser thermotherapy is associated with a cellular immune response. Br. J. Cancer 93, 435–440 (2005).
Challis, G. B. & Stam, H. J. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 29, 545–550 (1990). A collection of 744 cases of spontaneous regressions of cancer in humans, showing the theoretical justification for immunotherapy.
Hoption Cann, S. A., van Netten, J. P. & van Netten, C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad. Med. J. 79, 672–680 (2003).
Starnes, C. O. Coley's toxins in perspective. Nature 357, 11–12 (1992).
Coley, W. B. Treatment of inoperable malignant tumors with toxins of erysipelas and the bacillus prodigiosus. Trans. Am. Surg. Assn. 12, 183–212 (1894).
Bassi, P. BCG (Bacillus of Calmette Guerin) therapy of high-risk superficial bladder cancer. Surg. Oncol. 11, 77–83 (2002).
Zinkernagel, R. M. & Doherty, P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 27, 51–177 (1979).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Belardelli, F. & Ferrantini, M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 23, 201–208 (2002).
Kern, D. E., Klarnet, J. P., Jensen, M. C. & Greenberg, P. D. Requirement for recognition of class II molecules and processed tumor antigen for optimal generation of syngeneic tumor-specific class I-restricted CTL. J. Immunol. 136, 4303–4310 (1986).
Ahmad, M., Rees, R. C. & Ali, S. A. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol. Immunother. 53, 844–854 (2004).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev. Cancer 5, 263–274 (2005).
Agostinis, P., Buytaert, E., Breyssens, H. & Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 3, 721–729 (2004).
Moor, A. C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B 57, 1–13 (2000).
Plaetzer, K., Kiesslich, T., Oberdanner, C. B. & Krammer, B. Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection. Curr. Pharm. Des 11, 1151–1165 (2005).
Chen, B. et al. Photodynamic therapy with hypericin induces vascular damage and apoptosis in the RIF-1 mouse tumor model. Int. J. Cancer 98, 284–290 (2002).
Kaneko, T., Chiba, H., Yasuda, T. & Kusama, K. Detection of photodynamic therapy-induced early apoptosis in human salivary gland tumor cells in vitro and in a mouse tumor model. Oral Oncol. 40, 787–792 (2004).
Lilge, L., Portnoy, M. & Wilson, B. C. Apoptosis induced in vivo by photodynamic therapy in normal brain and intracranial tumour tissue. Br. J. Cancer 83, 1110–1117 (2000).
Magner, W. J. & Tomasi, T. B. Apoptotic and necrotic cells induced by different agents vary in their expression of MHC and costimulatory genes. Mol. Immunol. 42, 1033–1042 (2005).
Bartholomae, W. C. et al. T cell immunity induced by live, necrotic, and apoptotic tumor cells. J. Immunol. 173, 1012–1022 (2004).
Scheffer, S. R. et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int. J. Cancer 103, 205–211 (2003).
Shaif-Muthana, M. et al. Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res. 60, 6441–6447 (2000).
Zitvogel, L. et al. Immune response against dying tumor cells. Adv. Immunol. 84, 131–179 (2004).
Melcher, A., Gough, M., Todryk, S. & Vile, R. Apoptosis or necrosis for tumor immunotherapy: what's in a name? J. Mol. Med. 77, 824–833 (1999).
Yenari, M. A. et al. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. NY Acad. Sci. 1053, 74–83 (2005).
Korbelik, M., Sun, J. & Cecic, I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 65, 1018–1026 (2005).
Todryk, S. et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 163, 1398–1408 (1999).
Gomer, C. J. et al. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 56, 2355–2360 (1996). Demonstrates that PDT induces HSP expression both in vitro and in vivo.
Mitra, S., Goren, E. M., Frelinger, J. G. & Foster, T. H. Activation of heat shock protein 70 promoter with meso-tetrahydroxyphenyl chlorin photodynamic therapy reported by green fluorescent protein in vitro and in vivo. Photochem. Photobiol. 78, 615–622 (2003).
Verwanger, T. et al. Gene expression pattern following photodynamic treatment of the carcinoma cell line A-431 analysed by cDNA arrays. Int. J. Oncol. 21, 1353–1359 (2002).
Verrico, A. K., Haylett, A. K. & Moore, J. V. In vivo expression of the collagen-related heat shock protein HSP47, following hyperthermia or photodynamic therapy. Lasers Med. Sci. 16, 192–198 (2001).
Hanlon, J. G. et al. Induction of Hsp60 by Photofrin-mediated photodynamic therapy. J. Photochem. Photobiol. B 64, 55–61 (2001).
Gomer, C. J. et al. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 51, 6574–6579 (1991).
Nowis, D. et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 6 Feb 2006 (doi 10. 1038/sj. onc1209378).
Jiang, H. et al. Selective depletion of a thymocyte subset in vitro with an immunomodulatory photosensitizer. Clin. Immunol. 91, 178–187 (1999).
Jiang, H. et al. Selective action of the photosensitizer QLT0074 on activated human T lymphocytes. Photochem. Photobiol. 76, 224–231 (2002).
Boumedine, R. S. & Roy, D. C. Elimination of alloreactive T cells using photodynamic therapy. Cytotherapy 7, 134–143 (2005).
Hunt, D. W. & Levy, J. G. Immunomodulatory aspects of photodynamic therapy. Expert Opin. Investig. Drugs 7, 57–64 (1998).
Edstrom, D. W., Porwit, A. & Ros, A. M. Photodynamic therapy with topical 5-aminolevulinic acid for mycosis fungoides: clinical and histological response. Acta Derm. Venereol. 81, 184–188 (2001).
Steubing, R. W. et al. Activation of macrophages by Photofrin II during photodynamic therapy. J. Photochem. Photobiol. B 10, 133–145 (1991).
Evans, S. et al. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J. Natl Cancer Inst. 82, 34–39 (1990). Shows that PDT produces TNF α from macrophages.
Yamamoto, N. et al. Photodynamic immunopotentiation: in vitro activation of macrophages by treatment of mouse peritoneal cells with haematoporphyrin derivative and light. Eur. J. Cancer 27, 467–471 (1991).
Yamamoto, N. & Naraparaju, V. R. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 57, 2187–2192 (1997).
Korbelik, M. & Krosl, G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem. Photobiol. 60, 497–502 (1994).
Marshall, J. F., Chan, W. S. & Hart, I. R. Effect of photodynamic therapy on anti-tumor immune defenses: comparison of the photosensitizers hematoporphyrin derivative and chloro-aluminum sulfonated phthalocyanine. Photochem. Photobiol. 49, 627–632 (1989).
Gollnick, S. O. et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 88, 1772–1779 (2003). Describes the production of pro-inflammatory cytokines after PDT in vivo.
Yom, S. S. et al. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem. Photobiol. 78, 75–81 (2003).
Henderson, B. W. & Donovan, J. M. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 49, 6896–6900 (1989).
Henderson, B. W., Owczarczak, B., Sweeney, J. & Gessner, T. Effects of photodynamic treatment of platelets or endothelial cells in vitro on platelet aggregation. Photochem. Photobiol. 56, 513–521 (1992).
Fingar, V. H., Wieman, T. J. & Doak, K. W. Role of thromboxane and prostacyclin release on photodynamic therapy-induced tumor destruction. Cancer Res. 50, 2599–2603 (1990).
Balkwill, F., Charles, K. A. & Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217 (2005).
Sun, S. C. & Xiao, G. Deregulation of NF-κB and its upstream kinases in cancer. Cancer Metastasis Rev. 22, 405–422 (2003).
Baeuerle, P. A. & Henkel, T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12, 141–179 (1994).
Haefner, B. NF-κB: arresting a major culprit in cancer. Drug Discov. Today 7, 653–663 (2002).
Ryter, S. W. & Gomer, C. J. Nuclear factor κB binding activity in mouse L1210 cells following photofrin II-mediated photosensitization. Photochem. Photobiol. 58, 753–756 (1993). First report of PDT triggering NFκB signalling.
Kick, G. et al. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-κB DNA binding. Cancer Res. 55, 2373–2379 (1995).
Matroule, J. Y. et al. Pyropheophorbide-a methyl ester-mediated photosensitization activates transcription factor NF-κB through the interleukin-1 receptor-dependent signaling pathway. J. Biol. Chem. 274, 2988–3000 (1999).
Matroule, J. Y. et al. Role of nuclear factor-κB in colon cancer cell apoptosis mediated by aminopyropheophorbide photosensitization. Photochem. Photobiol. 70, 540–548 (1999).
Granville, D. J. et al. Nuclear factor-κB activation by the photochemotherapeutic agent verteporfin. Blood 95, 256–262 (2000).
Ferrario, A. et al. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy- mediated tumor response. Cancer Res. 62, 3956–3961 (2002).
Mitsuhashi, M. et al. Regulation of interleukin-12 gene expression and its anti-tumor activities by prostaglandin E2 derived from mammary carcinomas. J. Leukoc. Biol. 76, 322–332 (2004).
Makowski, M. et al. Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin. Cancer Res. 9, 5417–5422 (2003).
Volanti, C. et al. Distinct transduction mechanisms of cyclooxygenase 2 gene activation in tumour cells after photodynamic therapy. Oncogene 24, 2981–2991 (2005).
Hendrickx, N. et al. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J. Biol. Chem. 278, 52231–52239 (2003).
Gollnick, S. O. et al. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 57, 3904–3909 (1997).
Henderson, B. W. et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 64, 2120–2126 (2004).
Coutier, S. et al. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat. Res. 158, 339–345 (2002).
Sluiter, W., de Vree, W. J., Pietersma, A. & Koster, J. F. Prevention of late lumen loss after coronary angioplasty by photodynamic therapy: role of activated neutrophils. Mol. Cell. Biochem. 157, 233–238 (1996).
de Vree, W. J., Fontijne-Dorsman, A. N., Koster, J. F. & Sluiter, W. Photodynamic treatment of human endothelial cells promotes the adherence of neutrophils in vitro. Br. J. Cancer 73, 1335–1340 (1996).
Volanti, C. et al. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene 23, 8649–8658 (2004).
de Vree, W. J. et al. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 56, 2908–2911 (1996). Shows that neutrophils have a crucial role in the PDT response in vivo.
Krosl, G., Korbelik, M. & Dougherty, G. J. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer 71, 549–555 (1995).
Cecic, I., Parkins, C. S. & Korbelik, M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 74, 712–720 (2001).
Kajita, T. & Hugli, T. E. C5a-induced neutrophilia. A primary humoral mechanism for recruitment of neutrophils. Am. J. Pathol. 137, 467–477 (1990).
Cecic, I., Serrano, K., Gyongyossy-Issa, M. & Korbelik, M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett. 225, 215–223 (2005).
Sun, J., Cecic, I., Parkins, C. S. & Korbelik, M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 1, 690–695 (2002).
Cecic, I. & Korbelik, M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 183, 43–51 (2002).
Canti, G. et al. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anti-Cancer Drugs 5, 443–447 (1994).
Korbelik, M., Krosl, G., Krosl, J. & Dougherty, G. J. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 56, 5647–5652 (1996). One of the first reports to show T-cell mediated immune response against cancer after PDT.
Korbelik, M. & Dougherty, G. J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 59, 1941–1946 (1999).
Hendrzak-Henion, J. A. et al. Role of the immune system in mediating the antitumor effect of benzophenothiazine photodynamic therapy. Photochem. Photobiol. 69, 575–581 (1999).
Castano, A. P., Liu, Q. & Hamblin, M.R. A green fluorescent protein-expressing murine tumour but not its wild-type counterpart is cured by photodynamic therapy. Br. J. Cancer 94, 391–397 (2006).
Uenaka, A. & Nakayama, E. Murine leukemia RL male 1 and sarcoma Meth A antigens recognized by cytotoxic T lymphocytes (CTL). Cancer Sci. 94, 931–936 (2003).
Gollnick, S. O., Vaughan, L. & Henderson, B. W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 62, 1604–1608 (2002). Shows that PDT is especially effective in preparing vaccines from tumour cell lysates.
Korbelik, M. & Sun, J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 55, 900–909 (2005).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Krug, A. et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 (2001).
Supajatura, V. et al. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 167, 2250–2256 (2001).
Seya, T. et al. Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res. 23, 4369–4376 (2003).
Myers, R. C. et al. Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum in murine transitional cell carcinoma. Urology 33, 230–235 (1989).
Korbelik, M., Sun, J. & Posakony, J. J. Interaction between photodynamic therapy and BCG immunotherapy responsible for the reduced recurrence of treated mouse tumors. Photochem. Photobiol. 73, 403–409 (2001).
Korbelik, M. & Cecic, I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B 44, 151–158 (1998).
Uehara, M. et al. Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma. Cancer Immunol. Immunother. 49, 401–409 (2000).
Taylor, P. R. et al. The b-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876–3882 (2002).
Roeder, A. et al. Toll-like receptors as key mediators in innate antifungal immunity. Med. Mycol. 42, 485–498 (2004).
Krosl, G. & Korbelik, M. Potentiation of photodynamic therapy by immunotherapy: the effect of schizophyllan (SPG). Cancer Lett. 84, 43–49 (1994).
Chen, W. R. et al. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem. Photobiol. 81, 190–195 (2005).
Korbelik, M., Sun, J., Cecic, I. & Serrano, K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem. Photobiol. Sci. 3, 812–816 (2004).
Korbelik, M., Naraparaju, V. R. & Yamamoto, N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer 75, 202–207 (1997).
Bellnier, D. A. Potentiation of photodynamic therapy in mice with recombinant human tumor necrosis factor-a. J. Photochem. Photobiol. B. 8, 203–210 (1991).
Golab, J. et al. Potentiation of the anti-tumour effects of Photofrin-based photodynamic therapy by localized treatment with G-CSF. Br. J. Cancer 82, 1485–1491 (2000).
Krosl, G., Korbelik, M., Krosl, J. & Dougherty, G. J. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 56, 3281–3286 (1996).
North, R. J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155, 1063–1074 (1982).
Zagozdzon, R. & Golab, J. Immunomodulation by anticancer chemotherapy: more is not always better (review). Int. J. Oncol. 18, 417–424 (2001).
Castano, A. P. & Hamblin, M. R. Anti-tumor immunity generated by photodynamic therapy in a metastatic murine tumor model. Proc. SPIE 5695, 7–16 (2005).
Fu, S. et al. TGF-β induces Foxp3 + T-regulatory cells from CD4 + CD25- precursors. Am. J. Transplant 4, 1614–1627 (2004).
Wahl, S. M., Swisher, J., McCartney-Francis, N. & Chen, W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 76, 15–24 (2004).
Jalili, A. et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 10, 4498–4508 (2004).
Saji, H. et al. Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy. Clin. Cancer Res. 12, 2568–2574 (2006). The immune response produced by PDT and dendritic cells can regress a distant untreated tumour.
Korbelik, M. & Sun, J. Cancer treatment by photodynamic therapy combined with adoptive immunotherapy using genetically altered natural killer cell line. Int. J. Cancer 93, 269–274 (2001).
Hunt, D. W. & Levy, J. G. Immunomodulatory aspects of photodynamic therapy. Expert Opin. Investig. Drugs 7, 57–64 (1998).
Musser, D. A., Camacho, S. H., Manderscheid, P. A. & Oseroff, A. R. The anatomic site of photodynamic therapy is a determinant for immunosuppression in a murine model. Photochem. Photobiol. 69, 222–225 (1999).
Simkin, G. O., Tao, J. S., Levy, J. G. & Hunt, D. W. IL-10 contributes to the inhibition of contact hypersensitivity in mice treated with photodynamic therapy. J. Immunol. 164, 2457–2462 (2000). Describes PDT-induced immune suppression as evidenced by reduction of CHS.
Gollnick, S. O. et al. IL-10 does not play a role in cutaneous Photofrin photodynamic therapy- induced suppression of the contact hypersensitivity response. Photochem. Photobiol. 74, 811–816. (2001).
Musser, D. A. & Oseroff, A. R. Characteristics of the immunosuppression induced by cutaneous photodynamic therapy: persistence, antigen specificity and cell type involved. Photochem. Photobiol. 73, 518–524 (2001).
Ohtani, M., Kobayashi, Y. & Watanabe, N. Gene expression in the elicitation phase of guinea pig DTH and CHS reactions. Cytokine 25, 246–253 (2004).
Lou, P. J. et al. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer 91, 441–446 (2004).
Abdel-Hady, E. S. et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 61, 192–196. (2001). One of the few papers describing immune response after PDT in humans.
Shikowitz, M. J. et al. Clinical trial of photodynamic therapy with meso-tetra (hydroxyphenyl) chlorin for respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 131, 99–105 (2005).
Pasare, C. & Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036 (2003).
Acknowledgements
A.P.C. was supported by a US Department of Defense CDMRP Breast Cancer Research Grant. M.R.H. was supported by the US National Institutes of Health. We thank T. N. Demidova for help and advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Neutropaenia
-
A reduction in numbers of circulating neutrophils that predisposes to infection.
- Erysipelas
-
A skin disease caused by Streptococcus pyogenes.
- Antigen
-
A macromolecule (usually a protein or polysaccharide) that is perceived as foreign and stimulates an immune response.
- Major histocompatibility complex
-
Cell membrane proteins that bind short peptides and are recognized by T-cell receptors.
- Innate immune response
-
The immediately available nonspecific defence against invading pathogens, which consists of cellular (neutrophils, macrophages and natural killer cells) and non-cellular (complement and antibacterial peptides) arms.
- Adaptive immune response
-
An antigen-specific defence that develops with time, which consists of cellular (cytotoxic and helper T-lymphocytes) and humoral (B-lymphocytes and antibody) arms.
- Fluence
-
The light energy delivered per unit area (J cm−2).
- Fluence rate
-
The rate at which light energy is delivered per unit area (W cm−2).
- Antigen-presenting cells
-
Phagocytic cells such as dendritic cells, macrophages and B cells, which take up foreign antigens, and present then through major histocompatibility complex class II and express co-stimulatory molecules to ensure an effective T-cell response.
- Cross-presentation
-
The process by which exogenous antigens that would normally be presented by dendritic cells in the context of major histocompatibility complex (MHC) class II to CD4+ T cells are also presented in the context of MHC class I to CD8+ T cells.
- Complement
-
A group of proteins in serum that function with antibodies (classical pathway) or in response to microbial stimuli (alternative pathway) to achieve the destruction of foreign blood cells or bacteria.
- Toll-like receptors
-
First discovered in Drosophila, these represent a conserved set of pattern-recognition molecules that are triggered by motifs present on bacteria, viruses and fungi to initiate signalling which attracts and activates immune cells.
- Hapten
-
A small reactive molecule that can bind to host proteins and stimulate an immune response.
- Delayed type hypersensitivity
-
A delayed-onset, cytokine-induced localized inflammatory reaction characterized by a large influx of macrophages.
Rights and permissions
About this article
Cite this article
Castano, A., Mroz, P. & Hamblin, M. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 6, 535–545 (2006). https://doi.org/10.1038/nrc1894
Issue Date:
DOI: https://doi.org/10.1038/nrc1894
This article is cited by
-
Mesoporous silica coated spicules for photodynamic therapy of metastatic melanoma
Journal of Nanobiotechnology (2024)
-
The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy
Journal of Nanobiotechnology (2024)
-
Upconversion nanoparticles and their potential in the realm of biomedical sciences and theranostics
Journal of Nanoparticle Research (2024)
-
Controlling the biodistribution and clearance of nanomedicines
Nature Reviews Bioengineering (2023)
-
Metals to combat antimicrobial resistance
Nature Reviews Chemistry (2023)