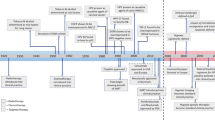
Abstract
Head and neck squamous-cell carcinoma (HNSCC) is the sixth most common cancer worldwide and, disappointingly, survival rates are not improving. Moreover, HNSCC has a severe impact on the quality of life of patients and survivors, and the significant morbidity subsequent to treatment often mandates long-term multidisciplinary care, which places significant financial pressures on the treating institution. Therefore, prevention and early diagnosis of high-risk pre-malignant lesions are high priorities for reducing deaths due to head and neck cancer. Recent advances have begun to elucidate the different aetiologies of HNSCCs in relation to previous pre-malignancies and to identify which pre-malignant lesions are likely to progress to malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lo, K. W., To, K. F. & Huang, D. P. Focus on nasopharyngeal carcinoma. Cancer Cell 5, 423–428 (2004).
Ries, L. A. G. et al. SEER Cancer Statistics Review, 1975–2001 (National Cancer Institute, Bethesda, 2004).
Parkin, D. M., Whelan, S. L., Ferlay, J., Teppo, L. & Thomas, D. B. Cancer Incidence in Five Continents, Vols. V–VIII, (IARC Scientific Publication, Lyon, 2003).
Mackenzie, J. et al. Increasing incidence of oral cancer amongst young persons: what is the aetiology? Oral Oncol. 36, 387–389 (2000).
Annertz, K. et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int. J. Cancer 101, 95–99 (2002).
Schantz, S. P. & Yu, G. P. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch. Otolaryngol. Head Neck Surg. 128, 268–274 (2002).
Rodriguez, T. et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 40, 207–213 (2004).
Chen, J. K., Katz, R. V. & Krutchkoff, D. J. Intraoral squamous cell carcinoma. Epidemiologic patterns in Connecticut from 1935 to 1985. Cancer 66, 1288–1296 (1990).
Cullen, J. W. et al. Health consequences of using smokeless tobacco: summary of the Advisory Committee's report to the Surgeon General. Public Health Rep. 101, 355–373 (1986).
Muir, C. S. & Kirk, R. Betel, tobacco, and cancer of the mouth. Br. J. Cancer 14, 597–608 (1960).
Winn, D. M. et al. Snuff dipping and oral cancer among women in the southern United States. N. Engl. J. Med. 304, 745–749 (1981).
Burns, J. E. et al. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br. J. Cancer 67, 1274–1284 (1993).
Chang, F. et al. Frequent mutations of p53 gene in oesophageal squamous cell carcinomas with and without human papillomavirus (HPV) involvement suggest the dominant role of environmental carcigones in oesophageal carcinogenesis. Br. J. Cancer 70, 346–351 (1994).
Brennan, J. A. et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 332, 712–717 (1995).
Seitz, H. K. et al. Alcohol and cancer. Alcohol Clin. Exp. Res. 25, 137S–143S (2001).
Poschl, G. & Seitz, H. K. Alcohol and cancer. Alcohol Alcohol 39, 155–165 (2004).
Hashibe, M., Ford, D. E. & Zhang, Z. F. Marijuana smoking and head and neck cancer. J. Clin. Pharmacol. 42, 103S–107S (2002).
Zhang, Z. F. et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 8, 1071–1078 (1999).
Macfarlane, G. J. et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur. J. Cancer B Oral Oncol. 31B, 181–187 (1995).
Macfarlane, G. J., Sharp, L., Porter, S. & Franceschi, S. Trends in survival from cancers of the oral cavity and pharynx in Scotland: a clue as to why the disease is becoming more common? Br. J. Cancer 73, 805–808 (1996).
Llewellyn, C. D., Linklater, K., Bell, J., Johnson, N. W. & Warnakulasuriya, K. A. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 39, 106–114 (2003).
Llewellyn, C. D., Linklater, K., Bell, J., Johnson, N. W. & Warnakulasuriya, S. An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncol. 40, 304–313 (2004).
Paz, I. B., Cook, N., Odom-Maryon, T., Xie, Y. & Wilczynski, S. P. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer 79, 595–604 (1997).
Gillison, M. L. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst. 92, 709–720 (2000).
Mork, J. et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 344, 1125–1131 (2001).
Smith, E. M. et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J. Natl Cancer Inst. 96, 449–455 (2004).
Herrero, R. et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J. Natl Cancer Inst. 95, 1772–1783 (2003).
Dahlstrom, K. R. et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin. Cancer Res. 9, 2620–2626 (2003).
Partridge, M., Pateromichelakis, S., Phillips, E., Emilion, G. & Langdon, J. Profiling clonality and progression in multiple premalignant and malignant oral lesions identifies a subgroup of cases with a distinct presentation of squamous cell carcinoma. Clin. Cancer Res. 7, 1860–1866 (2001).
Ringstrom, E. et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin. Cancer Res. 8, 3187–3192 (2002).
Gillison, M. L. & Lowy, D. R. A causal role for human papillomavirus in head and neck cancer. Lancet 363, 1488–1489 (2004).
Herrero, R. Human papillomavirus and cancer of the upper aerodigestive tract. J. Natl Cancer Inst. Monogr. 31, 47–51 (2003).
Braakhuis, B. J. et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J. Natl Cancer Inst. 96, 998–1006 (2004).
Klingelhutz, A. J., Foster, S. A. & McDougall, J. K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380, 79–82 (1996).
Cuthbert, A. P. et al. Telomerase repressor sequences on chromosome 3 and induction of permanent growth arrest in human breast cancer cells. J. Natl Cancer Inst. 91, 37–45 (1999).
de Vries, N., Van der Waal, I. & Snow, G. B. Multiple primary tumours in oral cancer. Int. J. Oral Maxillofac. Surg. 15, 85–87 (1986).
Mao, L., Hong, W. K. & Papadimitrakopoulou, V. A. Focus on head and neck cancer. Cancer Cell 5, 311–316 (2004).
Slaughter, D. P., Southwick, H. W. & Smejkal, W. “Field cancerisation” in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 6, 963–968 (1953).
Jang, S. J., Chiba, I., Hirai, A., Hong, W. K. & Mao, L. Multiple oral squamous epithelial lesions: are they genetically related? Oncogene 20, 2235–2242 (2001).
Spira, A. et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc. Natl Acad. Sci. USA 101, 10143–10148 (2004).
Hackett, N. R. et al. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am. J. Respir. Cell Mol. Biol. 29, 331–343 (2003).
Tabor, M. P. et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin. Cancer Res. 7, 1523–1532 (2001).
Tabor, M. P. et al. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am. J. Pathol. 161, 1051–1060 (2002).
Braakhuis, B. J., Tabor, M. P., Kummer, J. A., Leemans, C. R. & Brakenhoff, R. H. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 63, 1727–1730 (2003).
Braakhuis, B. J. et al. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck 24, 198–206 (2002).
Braakhuis, B. J., Leemans, C. R. & Brakenhoff, R. H. A genetic progression model of oral cancer: current evidence and clinical implications. J. Oral Pathol. Med. 33, 317–322 (2004).
Brennan, J. A. et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 332, 429–435 (1995).
van Houten, V. M. et al. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: a prospective study. Clin. Cancer Res. 10, 3614–3620 (2004).
Tabor, M. P. et al. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin. Cancer Res. 10, 3607–3613 (2004).
Partridge, M. et al. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin. Cancer Res. 9, 5287–5294 (2003).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Rheinwald, J. G. et al. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22, 5157–5172 (2002).
Romanov, S. R. et al. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 409, 633–637 (2001).
Muntoni, A. et al. Senescing oral dysplasias are not immortalized by ectopic expression of hTERT alone without other molecular changes, such as loss of INK4A and/or retinoic acid receptor-β: but p53 mutations are not necessarily required. Oncogene 22, 7804–7808 (2003).
Gordon, K. E. et al. High levels of telomere dysfunction bestow a selective disadvantage during the progression of human oral squamous cell carcinoma. Cancer Res. 63, 458–467 (2003).
Crawford, Y. G. et al. Histologically normal human mammary epithelia with silenced p16INK4a overexpress COX-2, promoting a premalignant program. Cancer Cell 5, 263–273 (2004).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Edington, K. G., Loughran, O. P., Berry, I. J. & Parkinson, E. K. Cellular immortality: a late event in the progression of human squamous cell carcinoma of the head and neck associated with p53 alteration and a high frequency of allele loss. Mol. Carcinog. 13, 254–265 (1995).
Loughran, O. et al. Evidence for the inactivation of multiple replicative lifespan genes in immortal human squamous cell carcinoma keratinocytes. Oncogene 14, 1955–1964 (1997).
Weber, R. G. et al. Recurrent chromosomal imbalances detected in biopsy material from oral premalignant and malignant lesions by combined tissue microdissection, universal DNA amplification, and comparative genomic hybridization. Am. J. Pathol. 153, 295–303 (1998).
Rosin, M. P. et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin. Cancer Res. 6, 357–362 (2000).
Mao, L. et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nature Med. 2, 682–685 (1996).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Shao, C. et al. Chromosome instability contributes to loss of heterozygosity in mice lacking p53. Proc. Natl Acad. Sci. USA 97, 7405–7410 (2000).
Field, J. K. et al. Allelotype of squamous cell carcinoma of the head and neck: fractional allele loss correlates with survival. Br. J. Cancer 72, 1180–1188 (1995).
Sudbo, J. et al. Cyclooxygenase-2 (COX-2) expression in high-risk premalignant oral lesions. Oral Oncol. 39, 497–505 (2003).
Shimada, Y. et al. Cell culture in esophageal squamous cell carcinoma and the association with molecular markers. Clin. Cancer Res. 9, 243–249 (2003).
Mao, L. et al. Telomerase activity in head and neck squamous cell carcinoma and adjacent tissues. Cancer Res. 56, 5600–5604 (1996).
Henson, J. D., Neumann, A. A., Yeager, T. R. & Reddel, R. R. Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 (2002).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Alonso, L. & Fuchs, E. Stem cells of the skin epithelium. Proc. Natl Acad. Sci. USA 100 (Suppl. 1), 11830–11835 (2003).
Holt, S. E., Wright, W. E. & Shay, J. W. Regulation of telomerase activity in immortal cell lines. Mol. Cell. Biol. 16, 2932–2939 (1996).
Ramirez, R. D., Wright, W. E., Shay, J. W. & Taylor, R. S. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J. Invest. Dermatol. 108, 113–117 (1997).
Dellambra, E. et al. Downregulation of 14-3-3σ prevents clonal evolution and leads to immortalization of primary human keratinocytes. J. Cell Biol. 149, 1117–1130 (2000).
Lindsey, J., McGill, N. I., Lindsey, L. A., Green, D. K. & Cooke, H. J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 256, 45–48 (1991).
Owens, D. M. & Watt, F. M. Contribution of stem cells and differentiated cells to epidermal tumours. Nature Rev. Cancer 3, 444–451 (2003).
Argyris, T. S. Tumor promotion by abrasion induced epidermal hyperplasia in the skin of mice. J. Invest. Dermatol. 75, 360–362 (1980).
Brown, K., Strathdee, D., Bryson, S., Lambie, W. & Balmain, A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol. 8, 516–524 (1998).
Barrandon, Y., Morgan, J. R., Mulligan, R. C. & Green, H. Restoration of growth potential in paraclones of human keratinocytes by a viral oncogene. Proc. Natl Acad. Sci. USA 86, 4102–4106 (1989).
Sudbo, J. et al. DNA content as a prognostic marker in patients with oral leukoplakia. N. Engl. J. Med. 344, 1270–1278 (2001).
Parkinson, E. K., Newbold, R. F. & Keith, W. N. The genetic basis of human keratinocyte immortalisation in squamous cell carcinoma development: the role of telomerase reactivation. Eur. J. Cancer 33, 727–734 (1997).
Yeager, T. R. et al. Overcoming cellular senescence in human cancer pathogenesis. Genes Dev. 12, 163–174 (1998).
Mashberg, A. Erythroplasia vs. leukoplasia in the diagnosis of early asymptomatic oral squamous carcinoma. N. Engl. J. Med. 297, 109–110 (1977).
Bouquot, J. E., Weiland, L. H. & Kurland, L. T. Leukoplakia and carcinoma in situ synchronously associated with invasive oral/oropharyngeal carcinoma in Rochester, Minn., 1935–1984. Oral Surg. Oral Med. Oral Pathol. 65, 199–207 (1988).
Hogewind, W. F., van der Waal, I., van der Kwast, W. A. & Snow, G. B. The association of white lesions with oral squamous cell carcinoma. A retrospective study of 212 patients. Int. J. Oral Maxillofac. Surg. 18, 163–164 (1989).
Califano, J. et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 56, 2488–2492 (1996).
Garnis, C., Baldwin, C., Zhang, L., Rosin, M. P. & Lam, W. L. Use of complete coverage array comparative genomic hybridization to define copy number alterations on chromosome 3p in oral squamous cell carcinomas. Cancer Res. 63, 8582–8585 (2003).
Garnis, C. et al. Novel regions of amplification on 8q distinct from the MYC locus and frequently altered in oral dysplasia and cancer. Genes Chromosom. Cancer 39, 93–98 (2004).
Garnis, C., Coe, B. P., Zhang, L., Rosin, M. P. & Lam, W. L. Overexpression of LRP12, a gene contained within an 8q22 amplicon identified by high-resolution array CGH analysis of oral squamous cell carcinomas. Oncogene 23, 2582–2586 (2004).
Garnis, C., Campbell, J., Zhang, L., Rosin, M. P. & Lam, W. L. OCGR array: an oral cancer genomic regional array for comparative genomic hybridization analysis. Oral Oncol. 40, 511–519 (2004).
Scully, C., Sudbo, J. & Speight, P. M. Progress in determining the malignant potential of oral lesions. J. Oral Pathol. Med. 32, 251–256 (2003).
Sudbo, J. & Reith, A. Which putatively pre-malignant oral lesions become oral cancers? Clinical relevance of early targeting of high-risk individuals. J. Oral Pathol. Med. 32, 63–70 (2003).
Kresty, L. A. et al. Alterations of p16INK4a and p14ARF in patients with severe oral epithelial dysplasia. Cancer Res. 62, 5295–5300 (2002).
Gallo, O., Santucci, M. & Franchi, A. Cumulative prognostic value of p16/CDKN2 and p53 oncoprotein expression in premalignant laryngeal lesions. J. Natl Cancer Inst. 89, 1161–1163 (1997).
Cruz, I. B. et al. p53 expression above the basal cell layer in oral mucosa is an early event of malignant transformation and has predictive value for developing oral squamous cell carcinoma. J. Pathol. 184, 360–368 (1998).
Rosin, M. P. et al. 3p14 and 9p21 loss is a simple tool for predicting second oral malignancy at previously treated oral cancer sites. Cancer Res. 62, 6447–6450 (2002).
Kim, J. et al. Chromosome polysomy and histological characteristics in oral premalignant lesions. Cancer Epidemiol. Biomarkers Prev. 10, 319–325 (2001).
Lee, J. J. et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin. Cancer Res. 6, 1702–1710 (2000).
Partridge, M. et al. A case-control study confirms that microsatellite assay can identify patients at risk of developing oral squamous cell carcinoma within a field of cancerization. Cancer Res. 60, 3893–3898 (2000).
Lee, J. I. et al. Loss of Fhit expression is a predictor of poor outcome in tongue cancer. Cancer Res. 61, 837–841 (2001).
Tanimoto, K. et al. Abnormalities of the FHIT gene in human oral carcinogenesis. Br. J. Cancer 82, 838–843 (2000).
Tai, S. K. et al. Loss of Fhit expression in head and neck squamous cell carcinoma and its potential clinical implication. Clin. Cancer Res. 10, 5554–5557 (2004).
Sudbo, J. et al. Abnormal DNA content predicts the occurrence of carcinomas in non-dysplastic oral white patches. Oral Oncol. 37, 558–565 (2001).
Sudbo, J. et al. The influence of resection and aneuploidy on mortality in oral leukoplakia. N. Engl. J. Med. 350, 1405–1413 (2004).
Sudbo, J. et al. Comparison of histological grading and large-scale genomic status (DNA ploidy) as prognostic tools in oral dysplasia. J. Pathol. 194, 303–310 (2001).
Remmerbach, T. W., Weidenbach, H., Hemprich, A. & Bocking, A. Earliest detection of oral cancer using non-invasive brush biopsy including DNA-image-cytometry: report on four cases. Anal. Cell Pathol. 25, 159–166 (2003).
Remmerbach, T. W. et al. Cytologic and DNA-cytometric early diagnosis of oral cancer. Anal. Cell Pathol. 22, 211–221 (2001).
Maraki, D., Becker, J. & Boecking, A. Cytologic and DNA-cytometric very early diagnosis of oral cancer. J. Oral Pathol. Med. 33, 398–404 (2004).
Burns, J. E. et al. The p53 status of cultured human premalignant oral keratinocytes. Br. J. Cancer 70, 591–595 (1994).
Lazarus, P. et al. Relationship between p53 mutation incidence in oral cavity squamous cell carcinomas and patient tobacco use. Carcinogenesis 17, 733–739 (1996).
Lazarus, P. et al. A low incidence of p53 mutations in pre-malignant lesions of the oral cavity from non-tobacco users. Int. J. Cancer 60, 458–463 (1995).
Qin, G. Z., Park, J. Y., Chen, S. Y. & Lazarus, P. A high prevalence of p53 mutations in pre-malignant oral erythroplakia. Int. J. Cancer 80, 345–348 (1999).
Ha, P. K. et al. A transcriptional progression model for head and neck cancer. Clin. Cancer Res. 9, 3058–3064 (2003).
McGregor, F. et al. Inappropriate retinoic acid receptor-β expression in oral dysplasias: correlation with acquisition of the immortal phenotype. Cancer Res. 57, 3886–3889 (1997).
McGregor, F. et al. Molecular changes associated with oral dysplasia progression and acquisition of immortality: potential for its reversal by 5-azacytidine. Cancer Res. 62, 4757–4766 (2002).
Gonzalez, H. E. et al. Identification of 9 genes differentially expressed in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 129, 754–759 (2003).
El-Naggar, A. K. et al. Differential expression profiling of head and neck squamous carcinoma: significance in their phenotypic and biological classification. Oncogene 21, 8206–8219 (2002).
Mendez, E. et al. Transcriptional expression profiles of oral squamous cell carcinomas. Cancer 95, 1482–1494 (2002).
Sok, J. C. et al. Tissue-specific gene expression of head and neck squamous cell carcinoma in vivo by complementary DNA microarray analysis. Arch. Otolaryngol. Head Neck Surg. 129, 760–770 (2003).
Hwang, D. et al. Genomic dissection for characterization of cancerous oral epithelium tissues using transcription profiling. Oral Oncol. 39, 259–268 (2003).
Alevizos, I. et al. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene 20, 6196–6204 (2001).
Leethanakul, C. et al. Gene expression profiles in squamous cell carcinomas of the oral cavity: use of laser capture microdissection for the construction and analysis of stage-specific cDNA libraries. Oral Oncol. 36, 474–483 (2000).
Leethanakul, C. et al. Distinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arrays. Oncogene 19, 3220–3224 (2000).
Al Moustafa, A. E. et al. Identification of genes associated with head and neck carcinogenesis by cDNA microarray comparison between matched primary normal epithelial and squamous carcinoma cells. Oncogene 21, 2634–2640 (2002).
Ginos, M. A. et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 64, 55–63 (2004).
Chung, C. H. et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5, 489–500 (2004).
Altorki, N. K., Subbaramaiah, K. & Dannenberg, A. J. COX-2 inhibition in upper aerodigestive tract tumors. Semin. Oncol. 31, 30–36 (2004).
Goodin, S. & Shiff, S. J. NSAIDs for the chemoprevention of oral cancer: promise or pessimism?: Commentary re J. L. Mulshine et al., randomized, double-blind, placebo-controlled, phase IIB trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in oropharyngeal leukoplakia. Clin. Cancer Res., 10: 1565–1573, 2004. Clin. Cancer Res. 10, 1561–1564 (2004).
Subbaramaiah, K., Cole, P. A. & Dannenberg, A. J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and-independent mechanisms. Cancer Res. 62, 2522–2530 (2002).
Minter, H. A., Eveson, J. W., Huntley, S., Elder, D. J. & Hague, A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin. Cancer Res. 9, 1885–1897 (2003).
Schroeder, C. P., Yang, P., Newman, R. A. & Lotan, R. Eicosanoid metabolism in squamous cell carcinoma cell lines derived from primary and metastatic head and neck cancer and its modulation by celecoxib. Cancer Biol. Ther. 3, 847–852 (2004).
Mulshine, J. L. et al. Randomized, double-blind, placebo-controlled phase IIb trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in oropharyngeal leukoplakia. Clin. Cancer Res. 10, 1565–1573 (2004).
Van Schooten, F. J. et al. Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol. Biomarkers Prev. 11, 167–175 (2002).
Singh, M., Krishanappa, R., Bagewadi, A. & Keluskar, V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol. 40, 591–596 (2004).
Kujan, O., Glenny, A. M., Duxbury, A. J., Thakker, N. & Sloan, P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst. Rev. CD004150 (2003).
Sidransky, D. Emerging molecular markers of cancer. Nature Rev. Cancer 2, 210–219 (2002).
Sanchez-Cespedes, M. et al. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 60, 892–895 (2000).
Rosas, S. L. et al. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 61, 939–942 (2001).
Spafford, M. F. et al. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin. Cancer Res. 7, 607–612 (2001).
Nawroz-Danish, H. et al. Microsatellite analysis of serum DNA in patients with head and neck cancer. Int. J. Cancer 111, 96–100 (2004).
Califano, J. et al. Detection of telomerase activity in oral rinses from head and neck squamous cell carcinoma patients. Cancer Res. 56, 5720–5722 (1996).
Freeman, A. et al. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin. Cancer Res. 5, 2121–2132 (1999).
Chatrath, P. et al. Aberrant expression of minichromosome maintenance protein-2 and Ki67 in laryngeal squamous epithelial lesions. Br. J. Cancer 89, 1048–1054 (2003).
Sidransky, D. et al. Serum protein MALDI profiling to distinguish upper aerodigestive tract cancer patients from control subjects. J. Natl Cancer Inst. 95, 1711–1717 (2003).
Wadsworth, J. T. et al. Serum protein profiles to identify head and neck cancer. Clin. Cancer Res. 10, 1625–1632 (2004).
Sun, S. Y. & Lotan, R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 41, 41–55 (2002).
Vieira, A. V., Schneider, W. J. & Vieira, P. M. Retinoids: transport, metabolism, and mechanisms of action. J. Endocrinol. 146, 201–207 (1995).
Lodi, G., Sardella, A., Bez, C., Demarosi, F. & Carrassi, A. Interventions for treating oral leukoplakia (Cochrane Review). in The Cochrane Library Vol. 2 (John Wiley & Sons, Chichester 2004).
Hong, W. K. et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N. Engl. J. Med. 315, 1501–1505 (1986).
Papadimitrakopoulou, V. A. et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch. Otolaryngol. Head Neck Surg. 125, 1083–1089 (1999).
Shin, D. M. et al. Combined interferon-α, 13-cis-retinoic acid, and α-tocopherol in locally advanced head and neck squamous cell carcinoma: novel bioadjuvant phase II trial. J. Clin. Oncol. 19, 3010–3017 (2001).
Hong, W. K. et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck [see comments]. N. Engl. J. Med. 323, 795–801 (1990).
Khuri, F. et al. Isotretinoin effects of head and neck cancer recurrence and second primary tumors. Proc. Am. Soc. Clin. Oncol. 22, 90 (2003).
van Zandwijk, N., Dalesio, O., Pastorino, U., de Vries, N. & van Tinteren, H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J. Natl Cancer Inst. 92, 977–986 (2000).
Ulanovski, D. et al. Expression of EGFR and Cerb-B2 as prognostic factors in cancer of the tongue. Oral Oncol. 40, 532–537 (2004).
Ang, K. K. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62, 7350–7356 (2002).
Chen, I. H. et al. Prognostic significance of EGFR and Her-2 in oral cavity cancer in betel quid prevalent area cancer prognosis. Br. J. Cancer 89, 681–686 (2003).
Kawakami, M. et al. Interleukin-13 receptor α2 chain in human head and neck cancer serves as a unique diagnostic marker. Clin. Cancer Res. 9, 6381–6388 (2003).
Estilo, C. L. et al. The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110α in squamous cell carcinoma of the oral tongue. Clin. Cancer Res. 9, 2300–2306 (2003).
Wada, S., Yue, L. & Furuta, I. Prognostic significance of p34cdc2 expression in tongue squamous cell carcinoma. Oral Oncol. 40, 164–169 (2004).
Mineta, H. et al. p53 mutation, but not p53 overexpression, correlates with survival in head and neck squamous cell carcinoma. Br. J. Cancer 78, 1084–1090 (1998).
Nathan, C. A., Sanders, K., Abreo, F. W., Nassar, R. & Glass, J. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: prognostic implications. Cancer Res. 60, 3599–3604 (2000).
Nogueira, C. P. et al. Inactivation of p53 and amplification of cyclin D1 correlate with clinical outcome in head and neck cancer. Laryngoscope 108, 345–350 (1998).
Geisler, S. A. et al. p16 and p53 protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin. Cancer Res. 8, 3445–3453 (2002).
Bova, R. J. et al. Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin. Cancer Res. 5, 2810–2819 (1999).
Ogi, K. et al. Aberrant methylation of multiple genes and clinicopathological features in oral squamous cell carcinoma. Clin. Cancer Res. 8, 3164–3171 (2002).
Knecht, R. et al. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 59, 2794–2797 (1999).
Lo Muzio, L. et al. Survivin expression in oral squamous cell carcinoma. Br. J. Cancer 89, 2244–2248 (2003).
Chang, H. W., Chow, V., Lam, K. Y., Wei, W. I. & Yuen, A. Loss of E-cadherin expression resulting from promoter hypermethylation in oral tongue carcinoma and its prognostic significance. Cancer 94, 386–392 (2002).
Lim, S. C. et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin. Cancer Res. 10, 166–172 (2004).
Chow, V. et al. A comparative study of the clinicopathological significance of E-cadherin and catenins (α, β, γ) expression in the surgical management of oral tongue carcinoma. J. Cancer Res. Clin. Oncol. 127, 59–63 (2001).
Moriyama-Kita, M. et al. Correlation of S100A4 expression with invasion and metastasis in oral squamous cell carcinoma. Oral Oncol. 40, 496–500 (2004).
Gonzalez-Moles, M. A. et al. Adhesion molecule CD44 as a prognostic factor in tongue cancer. AntiCancer Res. 23, 5197–5202 (2003).
Kosunen, A. et al. Reduced expression of hyaluronan is a strong indicator of poor survival in oral squamous cell carcinoma. Oral Oncol. 40, 257–263 (2004).
Katayama, A. et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin. Cancer Res. 10, 634–640 (2004).
Kobayashi, H. et al. Clinical significance of cellular distribution of moesin in patients with oral squamous cell carcinoma. Clin. Cancer Res. 10, 572–580 (2004).
Uehara, M. et al. Expression of vascular endothelial growth factor and prognosis of oral squamous cell carcinoma. Oral Oncol. 40, 321–325 (2004).
Shintani, S. et al. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 40, 13–20 (2004).
Beasley, N. J. et al. Hypoxia-inducible factors HIF-1α and HIF-2α in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 62, 2493–2497 (2002).
Miyazawa, J., Mitoro, A., Kawashiri, S., Chada, K. K. & Imai, K. Expression of mesenchyme-specific gene HMGA2 in squamous cell carcinomas of the oral cavity. Cancer Res. 64, 2024–2029 (2004).
Delilbasi, C. B., Okura, M., Iida, S. & Kogo, M. Investigation of CXCR4 in squamous cell carcinoma of the tongue. Oral Oncol. 40, 154–157 (2004).
Liao, C. T. et al. Telomerase as an independent prognostic factor in head and neck squamous cell carcinoma. Head Neck 26, 504–512 (2004).
Chang, B. W. et al. Prognostic significance of cyclooxygenase-2 in oropharyngeal squamous cell carcinoma. Clin. Cancer Res. 10, 1678–1684 (2004).
Yanagawa, T. et al. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinomas. Oral Oncol. 40, 21–27 (2004).
Acknowledgements
The work of P.R.H. and E.K.P. is supported by Cancer Research UK. K.D.H. is a clinical lecturer in oral pathology at the University of Glasgow. The authors' work is the product of a longstanding collaboration with clinical colleagues and pathologists in Glasgow, particularly G. MacDonald and D. Felix at the Glasgow Dental Hospital and School; D. Soutar at the Plastic Surgery Unit at Glasgow Royal Infirmary; and L. Clark at the Southern General Hospital, Glasgow. We are grateful to G. MacDonald and colleagues at the Beatson Institute (M. Frame, D. Gillespie and B. Ozanne) for constructive comments on the manuscript. We are also grateful to L. Batti at the Scottish Cancer Registry, Information and Statistics Division, Edinburgh, for supplying the Scottish and European oral cancer incidence statistics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Hunter, K., Parkinson, E. & Harrison, P. Profiling early head and neck cancer. Nat Rev Cancer 5, 127–135 (2005). https://doi.org/10.1038/nrc1549
Issue Date:
DOI: https://doi.org/10.1038/nrc1549
This article is cited by
-
Identification of CT-based non-invasive radiomic biomarkers for overall survival prediction in oral cavity squamous cell carcinoma
Scientific Reports (2023)
-
Is adrenomedullin upregulation due to apical periodontitis independent of periodontal disease?
Odontology (2023)
-
Clinical practice guidelines for the management of recurrent head and neck cancer: a systematic review and quality appraisal
European Archives of Oto-Rhino-Laryngology (2023)
-
HPV-mediated regulation of SMAD4 modulates the DNA damage response in head and neck cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
Serum Survivin in Oral Submucosal Fibrosis and Squamous Cell Carcinoma
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)