Key Points
-
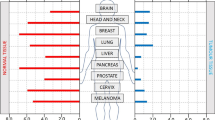
A characteristic feature of solid tumours is the presence of cells at very low oxygen tensions. These hypoxic cells confer radiotherapy and chemotherapy resistance to the tumours, as well as selecting for a more malignant phenotype.
-
These hypoxic cells, however, provide a tumour-specific targeting strategy for therapy, and four approaches are being investigated: prodrugs activated by hypoxia; hypoxia-selective gene therapy; targeting the hypoxia-inducible factor 1 (HIF-1) transcription factor; and the use of recombinant obligate anaerobic bacteria.
-
Tirapazamine is the prototype hypoxia-activated prodrug. Its toxic metabolite, a highly reactive radical that is present at higher concentrations under hypoxia, selectively kills the resistant hypoxic cells in tumours. This makes the tumours much more sensitive to treatment with conventional chemotherapy and radiotherapy.
-
Several other hypoxia-activated prodrugs, including AQ4N, NLCQ-1 and dinitrobenzamide mustards, are in preclinical or early clinical development.
-
Hypoxia-activated gene therapy using hypoxia-specific promoters provides selective transcription of enzymes that can convert prodrugs into toxic drugs. The efficacy of this approach has been shown in animal models, but clinical testing must await better systemic delivery of vectors to hypoxic cells.
-
Targeting HIF-1 is a third strategy. This protein is stabilized under hypoxic conditions and promotes the survival of tumour cells under hypoxic conditions. Several strategies to inactivate or to exploit this unique protein in tumours are being investigated at the preclinical level.
-
Finally, using recombinant non-pathogenic clostridia — an obligate anaerobe that colonizes tumour necrosis after systemic administration — is another strategy to exploit the unique physiology of solid tumours. This approach has demonstrated considerable preclinical efficacy.
Abstract
Solid tumours contain regions at very low oxygen concentrations (hypoxia), often surrounding areas of necrosis. The cells in these hypoxic regions are resistant to both radiotherapy and chemotherapy. However, the existence of hypoxia and necrosis also provides an opportunity for tumour-selective therapy, including prodrugs activated by hypoxia, hypoxia-specific gene therapy, targeting the hypoxia-inducible factor 1 transcription factor, and recombinant anaerobic bacteria. These strategies could turn what is now an impediment into a significant advantage for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thomlinson, R. H. & Gray, L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955).
Gray, L. H., Conger, A. D., Ebert, M., Hornsey, S. & Scott, O. C. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 26, 638–648 (1953). References 1 and 2 are classic papers describing both the general nature of the oxygen effect in reducing radiation sensitivity (reference 2) and the fact that the hypoxic cells almost certainly are present in human tumours (reference 1).
Brown, J. M. Clinical trials of radiosensitizers: what should we expect? Int. J. Radiat. Oncol. Biol. Phys. 10, 425–429 (1984).
Overgaard, J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol. Res. 6, 509–518 (1994).
Vaupel, P., Schlenger, K., Knoop, C. & Hockel, M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 51, 3316–3322 (1991).
Nordsmark, M., Overgaard, M. & Overgaard, J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 41, 31–40 (1996).
Brizel, D. M., Dodge, R. K., Clough, R. W. & Dewhirst, M. W. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother. Oncol. 53, 113–117 (1999).
Tannock, I. F. Conventional cancer therapy: promise broken or promise delayed? Lancet 351 (Suppl. 2), 9–16 (1998).
Durand, R. E. The influence of microenvironmental factors during cancer therapy. In vivo 8, 691–702 (1994).
Tannock, I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br. J. Cancer 22, 258–273 (1968).
Teicher, B. A., Lazo, J. S. & Sartorelli, A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 41, 73–81 (1981).
Batchelder, R. M., Wilson, W. R., Hay, M. P. & Denny, W. A. Oxygen dependence of the cytotoxicity of the enediyne anti-tumour antibiotic esperamicin A1. Br. J. Cancer Suppl. 27, S52–S56 (1996).
Comerford, K. M. et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62, 3387–3394 (2002).
Wartenberg, M. et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 17, 503–505 (2003).
Graeber, T. G. et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379, 88–91 (1996). Describes how hypoxia in tumours selects against wild-type p53 by causing apoptosis in these cells.
Yuan, J. & Glazer, P. M. Mutagenesis induced by the tumor microenvironment. Mutat. Res. 400, 439–446 (1998).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Pennacchietti, S. et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3, 347–361 (2003).
Rofstad, E. K. Microenvironment-induced cancer metastasis. Int. J. Radiat. Biol. 76, 589–605 (2000).
Subarsky, P. & Hill, R. P. The hypoxic tumour microenvironment and metastatic progression. Clin. Exp. Metastasis 20, 237–250 (2003).
Hockel, M. et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 56, 4509–4515 (1996).
Brizel, D. M. et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 56, 941–943 (1996).
Zeman, E. M., Brown, J. M., Lemmon, M. J., Hirst, V. K. & Lee, W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int. J. Radiat. Oncol. Biol. Phys. 12, 1239–1242 (1986). The initial paper describing the hypoxic selectivity of tirapazamine for cell killing.
Brown, J. M. & Lemmon, M. J. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 50, 7745–7749 (1990).
Brown, J. M. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br. J. Cancer 67, 1163–1170 (1993).
Daniels, J. S. & Gates, K. S. DNA cleavage by the antitumor agent 3-amino-1,2,4-benzotriazine 1,4-dioxide (SR4233): Evidence for involvement of hydroxyl radical. J. Am. Chem. Soc. 118, 3380–3385 (1996).
Zagorevskii, D. et al. A mass spectrometry study of tirapazamine and its metabolites. insights into the mechanism of metabolic transformations and the characterization of reaction intermediates. J. Am. Soc. Mass Spectrom. 14, 881–892 (2003).
Anderson, R. F., Shinde, S. S., Hay, M. P., Gamage, S. A. & Denny, W. A. Activation of 3-amino-1,2,4-benzotriazine 1,4-dioxide antitumor agents to oxidizing species following their one-electron reduction. J. Am. Chem. Soc. 125, 748–756 (2003).
Peters, K. B. & Brown, J. M. Tirapazamine: a hypoxia-activated topoisomerase II poison. Cancer Res. 62, 5248–5253 (2002).
Dorie, M. J. & Brown, J. M. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res. 53, 4633–4636 (1993). In vivo data showing that tirapazamine and cisplatin have a marked hypoxia and schedule-dependent synergism.
Kovacs, M. S. et al. Cisplatin anti-tumour potentiation by tirapazamine results from a hypoxia-dependent cellular sensitization to cisplatin. Br. J Cancer 80, 1245–1251 (1999).
von Pawel, J. et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. J. Clin. Oncol. 18, 1351–1359 (2000). Clinical data showing the efficacy of tirapazamine in combination with cisplatin in a randomized multicentre Phase III trial.
Rischin, D. et al. Preliminary results of TROG 98.02—a randomized phase II study of 5-fluorouracil, cisplatin and radiation versus tirapazamine, cisplatin and radiation for advanced squamous cell carcinoma of the head and neck. Proc. Am. Soc. Clin. Oncol. 22, A1992 (2003).
Patterson, L. H. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev. 12, 119–134 (1993).
Patterson, L. H. Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab. Rev. 34, 581–592 (2002).
Patterson, L. H. & Murray, G. I. Tumour cytochrome P450 and drug activation. Curr. Pharm. Des. 8, 1335–1347 (2002).
Patterson, L. H. & McKeown, S. R. AQ4N: a new approach to hypoxia-activated cancer chemotherapy. Br. J. Cancer 83, 1589–1593 (2000).
Patterson, L. H. et al. Enhancement of chemotherapy and radiotherapy of murine tumours by AQ4N, a bioreductively activated anti-tumour agent. Br. J. Cancer 82, 1984–1990 (2000). Demonstration of potentiation of radiation and chemotherapy by the bioreductive agent AQ4N.
Adams, G. E. & Stratford, I. J. Bioreductive drugs for cancer therapy: the search for tumor specificity. Int. J. Radiat. Oncol. Biol. Phys. 29, 231–238 (1994).
Brown, J. M. & Siim, B. G. Hypoxia-specific cytotoxins in cancer therapy. Semin. Rad. Onc. 6, 22–36 (1996).
de Groot, F. M., Damen, E. W. & Scheeren, H. W. Anticancer prodrugs for application in monotherapy: targeting hypoxia, tumor-associated enzymes, and receptors. Curr. Med. Chem. 8, 1093–1122 (2001).
Denny, W. A. & Wilson, W. R. Bioreducible mustards: a paradigm for hypoxia-selective prodrugs of diffusible cytotoxins (HPDCs). Cancer Metastasis Rev. 12, 135–151 (1993).
Naylor, M. A. & Thomson, P. Recent advances in bioreductive drug targeting. Mini Rev. Med. Chem. 1, 17–29 (2001).
Rauth, A. M., Melo, T. & Misra, V. Bioreductive therapies: an overview of drugs and their mechanisms of action. Int. J. Radiat. Oncol. Biol. Phys. 42, 755–762 (1998).
Rockwell, S. Use of hypoxia-directed drugs in the therapy of solid tumors. Semin. Oncol. 19, 29–40 (1992).
Workman, P. & Stratford, I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 12, 73–82 (1993).
Papadopoulou, M. V. & Bloomer, W. D. NLCQ-1 (NSC 709257): exploiting hypoxia with a weak DNA-intercalating bioreductive drug. Clin. Cancer Res. 9, 5714–5720 (2003).
Hicks, K. O., Pruijn, F. B., Baguley, B. C. & Wilson, W. R. Extravascular transport of the DNA intercalator and topoisomerase poison N-[2-(Dimethylamino)ethyl]acridine-4-carboxamide (DACA): diffusion and metabolism in multicellular layers of tumor cells. J. Pharmacol. Exp. Ther. 297, 1088–1098 (2001).
Delahoussaye, Y. M., Hay, M. P., Pruijn, F. B., Denny, W. A. & Brown, J. M. Improved potency of the hypoxic cytotoxin tirapazamine by DNA-targeting. Biochem. Pharmacol. 65, 1807–1815 (2003).
Hay, M. P. et al. DNA-targeted 1,2,4-benzotriazine 1,4-dioxides as hypoxia–selective analogues of tirapazamine. J. Med. Chem. 47, 475–488 (2004).
Denny, W. A., Wilson, W. R. & Hay, M. P. Recent developments in the design of bioreductive drugs. Br. J. Cancer Suppl. 27, S32–S38 (1996).
Koch, C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 53, 3992–3997 (1993).
Hicks, K. O., Siim, B. G., Pruijn, F. B. & Wilson, W. R. Oxygen dependence of the metabolic activation and cytotoxicity of tirapazamine: Implications for exptravascular transport and activity in tumors. Radiat. Res. (in the press).
Marshall, R. S. & Rauth, A. M. Oxygen and exposure kinetics as factors influencing the cytotoxicity of porfiromycin, a mitomycin C analogue, in Chinese hamster ovary cells. Cancer Res. 48, 5655–5659 (1988).
Siim, B. G., Atwell, G. J. & Wilson, W. R. Oxygen dependence of the cytotoxicity and metabolic activation of 4-alkylamino-5-nitroquinoline bioreductive drugs. Br. J. Cancer 70, 596–603 (1994).
Wilson, W. R., Moselen, J. W., Cliffe, S., Denny, W. A. & Ware, D. C. Exploiting tumor hypoxia through bioreductive release of diffusible cytotoxins: the cobalt(III)-nitrogen mustard complex SN 24771. Int. J. Radiat. Oncol. Biol. Phys. 29, 323–327 (1994).
Wouters, B. G. & Brown, J. M. Cells at intermediate oxygen levels can be more important than the 'hypoxic fraction' in determining tumor response to fractionated radiotherapy. Radiat. Res. 147, 541–550 (1997).
Lee, A. E. & Wilson, W. R. Hypoxia-dependent retinal toxicity of bioreductive anticancer prodrugs in mice. Toxicol. Appl. Pharmacol. 163, 50–59 (2000).
Allalunis, M. J., Chapman, J. D. & Turner, A. R. Identification of a hypoxic population of bone marrow cells. Int. J. Radiat. Oncol. Biol. Phys. 9, 227–232 (1983).
Cipolleschi, M. G., Dello Sbarba, P. & Olivotto, M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82, 2031–2037 (1993).
Siim, B. G., Denny, W. A. & Wilson, W. R. Nitro reduction as an electronic switch for bioreductive drug activation. Oncol. Res. 9, 357–369 (1997).
Helsby, N. A. et al. Effect of nitroreduction on the alkylating reactivity and cytotoxicity of the 2,4-dinitrobenzamide-5-aziridine CB 1954 and the corresponding nitrogen mustard SN 23862: distinct mechanisms of bioreductive activation. Chem. Res. Toxicol. 16, 469–478 (2003).
Wilson, W. R. et al. Quantitation of bystander effects in nitroreductase suicide gene therapy using three-dimensional cell cultures. Cancer Res. 62, 1425–1432 (2002). Demonstrates the use of three-dimensional cell cultures to show bystander effects from GDEPT.
Borch, R. F. et al. Synthesis and evaluation of nitroheterocyclic phosphoramidates as hypoxia-selective alkylating agents. J. Med. Chem. 43, 2258–2265 (2000).
Tercel, M. et al. Hypoxia-selective antitumor agents. 16. Nitroarylmethyl quaternary salts as bioreductive prodrugs of the alkylating agent mechlorethamine. J. Med. Chem. 44, 3511–3522 (2001).
Wilson, W. R., Moselen, J. W., Cliffe, S., Denny, W. A. & Ware, D. C. Exploiting tumor hypoxia through bioreductive release of diffusible cytotoxins: the cobalt(III)-nitrogen mustard complex SN 24771. Int. J. Radiat. Oncol. Biol. Phys. 29, 323–327 (1994).
Everett, S. A. et al. Modifying rates of reductive elimination of leaving groups from indolequinone prodrugs: a key factor in controlling hypoxia-selective drug release. Biochem. Pharmacol. 63, 1629–1639 (2002).
Wilson, W. R., Tercel, M., Anderson, R. F. & Denny, W. A. Radiation-activated prodrugs as hypoxia-selective cytotoxins: model studies with nitroarylmethyl quaternary salts. Anticancer Drug Des. 13, 663–685 (1998).
Kriste, A. G., Tercel, M., Anderson, R. F., Ferry, D. M. & Wilson, W. R. Pathways of reductive fragmentation of heterocyclic nitroarylmethyl quaternary ammonium prodrugs of mechlorethamine. Radiat. Res. 158, 753–762 (2002).
Ahn, G., Ware, D. C., Denny, W. A. & Wilson, W. R. Optimization of the auxiliary ligand shell of cobalt(III)(8-hydroxyquinoline) complexes as model hypoxia-selective radiation–activated prodrugs. Radiat. Res. (in the press).
Shibamoto, Y., Zhou, L., Hatta, H., Mori, M. & Nishimoto, S. A novel class of antitumor prodrug, 1-(2′-oxopropyl)-5-fluorouracil (OFU001), that releases 5-fluorouracil upon hypoxic irradiation. Jpn J. Cancer Res. 91, 433–438 (2000).
Zhong, H. et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 59, 5830–5835 (1999).
Talks, K. L. et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411–421 (2000).
Shibata, T., Akiyama, N., Noda, M., Sasai, K. & Hiraoka, M. Enhancement of gene expression under hypoxic conditions using fragments of the human vascular endothelial growth factor and the erythropoietin genes. Int. J. Radiat. Oncol. Biol. Phys. 42, 913–916 (1998).
Greco, O. & Dachs, G. U. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J. Cell Physiol. 187, 22–36 (2001).
Binley, K. et al. Hypoxia-mediated tumour targeting. Gene Ther. 10, 540–549 (2003).
Shibata, T., Giaccia, A. J. & Brown, J. M. Hypoxia-inducible regulation of a prodrug-activating enzyme for tumor-specific gene therapy. Neoplasia 4, 40–48 (2002).
Patterson, A. V. et al. Oxygen-sensitive enzyme-prodrug gene therapy for the eradication of radiation-resistant solid tumours. Gene Ther. 9, 946–954 (2002).
Trinh, Q. T., Austin, E. A., Murray, D. M., Knick, V. C. & Huber, B. E. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5- fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 55, 4808–4812 (1995).
McCarthy, H. O. et al. Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther. 10, 40–48 (2003).
Griffiths, L. et al. The macrophage: a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 7, 255–262 (2000).
Burke, B. et al. Expression of HIF-1α by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J. Pathol. 196, 204–212 (2002).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Giaccia, A., Siim, B. G. & Johnson, R. S. HIF-1 as a target for drug development. Nature Rev. Drug Discov. 2, 803–811 (2003).
Semenza, G. L. Involvement of hypoxia-inducible factor 1 in human cancer. Intern. Med. 41, 79–83 (2002).
Kung, A. L., Wang, S., Klco, J. M., Kaelin, W. G. & Livingston, D. M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nature Med. 6, 1335–1340 (2000).
Rapisarda, A. et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 62, 4316–4324 (2002).
Sun, X. et al. Gene transfer of antisense hypoxia inducible factor-1α enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 8, 638–645 (2001).
Mabjeesh, N. J. et al. Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer Res. 62, 2478–2482 (2002).
Mabjeesh, N. J. et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3, 363–375 (2003).
Yeo, E. J. et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J. Natl Cancer Inst. 95, 516–525 (2003).
Lemmon, M. J. et al. Anaerobic bacteria as a gene delivery system to tumors. Proc. Am. Assoc. Cancer Res. 35, 374 (1994).
Fox, M. E. et al. Anaerobic bacteria as a delivery system for cancer gene therapy: activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther. 3, 173–178 (1996).
Lemmon, M. L. et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 4, 791–796 (1997).
Malmgren, R. A. & Flanigan, C. C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 15, 473–478 (1955).
Möse, J. R. & Möse, G. Onkolyseversuche mit apathogenen anaeroben Sporenbildern am Ehrlich Tumor des Maus. Z. Krebsforsch 63, 63–74 (1959).
Möse, J. R. & Möse, G. Oncolysis by clostridia. I. Activity of Clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 24, 212–216 (1964).
Thiele, E. H., Arison, R. N. & Boxer, G. E. Oncolysis by clostridia. III. Effects of clostridia and chemotherapeutic agents on rodent tumors. Cancer Res. 24 (1964).
Engelbart, K. & Gericke, D. Oncolysis by clostridia V. Transplanted tumors of the hamster. Cancer Res. 24, 239–243 (1964).
Carey, R. W., Holland, J. F., Whang, H. Y., Neter, E. & Bryant, B. Clostridial oncolysis in man. Europ. J. Cancer 3, 37–46 (1967).
Heppner, F. & Mose, J. R. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic clostridium. Acta Neuro. 12, 123–125 (1978).
Heppner, F., Mose, J., Ascher, P. W. & Walter, G. Oncolysis of malignant gliomas of the brain. 13th Int. Cong. Chemother. 226, 38–45 (1983).
Pawelek, J. M., Low, K. B. & Bermudes, D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 57, 4537–4544 (1997).
Toso, J. F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20, 142–152 (2002).
Liu, S. C., Minton, N. P., Giaccia, A. J. & Brown, J. M. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 9, 291–296 (2002). First data demonstrating in vivo efficacy of CDEPT.
Bridgewater, J. A. et al. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur. J. Cancer 31A, 2362–2370 (1995).
Minton, N. P. Clostridia in cancer therapy. Nat. Rev. Microbiol. 1, 237–242 (2003).
Martin, J. et al. Antibody-directed enzyme prodrug therapy: pharmacokinetics and plasma levels of prodrug and drug in a phase I clinical trial. Cancer Chemother. Pharmacol. 40, 189–201 (1997).
Joseph, W. R. et al. Stimulation of tumors to synthesize tumor necrosis factor-α in situ using 5,6-dimethylxanthenone-4-acetic acid: a novel approach to cancer therapy. Cancer Res. 59, 633–638 (1999).
Zhao, L., Ching, L. M., Kestell, P. & Baguley, B. C. The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. Br. J. Cancer 87, 465–470 (2002).
Ching, L. M. et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-Dimethylxanthenone-4-acetic acid. Br. J. Cancer 86, 1937–1942 (2002).
Galbraith, S. M. et al. Effects of 5,6-dimethylxanthenone-4-acetic acid on human tumor microcirculation assessed by dynamic contrast-enhanced magnetic resonance imaging. J. Clin. Oncol. 20, 3826–3840 (2002).
Theys, J. et al. Improvement of Clostridium tumour targeting vectors evaluated in rat rhabdomyosarcomas. FEMS Immunol. Med. Microbiol. 30, 37–41 (2001).
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W. & Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl Acad. Sci. USA 98, 15155–15160 (2001).
Hicks, K. O., Pruijn, F. B., Sturman, J. R., Denny, W. A. & Wilson, W. R. Multicellular resistance to tirapazamine is due to restricted extravascular transport in HT29 multicellular layer cultures: A pharmacokinetic/pharmacodynamic study. Cancer Res. 63, 5970–5977 (2003).
Helsby, N. A., Ferry, D. M., Patterson, A. V., Pullen, S. M. & Wilson, W. R. 2–amino metabolites are key mediatiors of CB 1954 and SN 23862 bystander effects in nitroreductase GDEPT. Br. J.Cancer 90, 1084–1092 (2004).
Bussink, J., Kaanders, J. H. A. M. & van der Kogel, A. J. Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exdogenous and endogenous hypoxic cell markers. Radiother. Oncol. 67, 3–15 (2003).
Cobb, L. M. et al. 2,4-Dinitro-5-ethyleneiminobenzamide (CB 1954): A potent and selective inhibitor of the growth of the Walker carcinoma 256. Biochem. Pharmacol. 18, 1519–1527 (1969).
Knox, R. J., Friedlos, F., Jarman, M. & Roberts, J. J. A new cytotoxic, DNA interstrand crosslinking agent, 5-(Aziridin-1-YL)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(Aziridin-1-YL)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem. Pharm. 37, 4661–4669 (1988).
Knox, R. J. et al. The nitroreductase enzyme in Walker cells that activates 5-(Aziridin-1-YL)-2,4-dinitrobenzamide (CB 1954) to 5-(Aziridin-1-YL)-4-hydroxylamino-2-nitrobenzamide is a form of NAD(P)H dehydrogenase (quinone) (EC 1. 6. 99. 2). Biochem. Pharmacol. 37, 4671–4677 (1988).
Anlezark, G. M. et al. The bioactivation of 5-(Aziridin-1-YL)-2,4-dinitrobenzamide (CB 1954)-I Purification and properties of a nitroreductase enzyme from Escherichia Coli: A potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT). Biochem. Pharmacol. 44, 2289–2295 (1992).
Chung-Faye, G. et al. Virus-directed, enzyme prodrug therapy with nitroimidazole reductase: a phase I and pharmacokinetic study of its prodrug, CB1954. Clin. Cancer Res. 7, 2662–2668 (2001).
Stratford, I. J., Williamson, C., Hoe, S. & Adams, G. E. Radiosensitizing and cytotoxicity studies with CB 1954 (2,4-dinitro-5-aziridinylbenzamide). Radiat. Res. 88, 502–509 (1981).
Palmer, B. D., Wilson, W. R., Cliffe, S. & Denny, W. A. Hypoxia-selective antitumor agents. 5. Synthesis of water-soluble nitroaniline mustards with selective cytotoxicity for hypoxic mammalian cells. J. Med. Chem. 35, 3214–3222 (1992).
Brown, J. M. & Giaccia, A. J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 58, 1408–1416 (1998).
Wouters, B. G. et al. Mitochondrial dysfunction after aerobic exposure to the hypoxic cytotoxin tirapazamine. Cancer Res. 61, 145–152 (2001).
Aebersold, D. M. et al. Expression of hypoxia-inducible factor-1α: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 61, 2911–2916 (2001). Shows that HIF-1 can be used as an endogenous marker of tumour hypoxia to predict response to radiotherapy.
Rampling, R., Cruickshank, G., Lewis, A. D., Fitzsimmons, S. A. & Workman, P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 29, 427–431 (1994).
Collingridge, D. R., Piepmeier, J. M., Rockwell, S. & Knisely, J. P. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother. Oncol. 53, 127–131 (1999).
Nordsmark, M., Bentzen, S. M. & Overgaard, J. Measurement of human tumour oxygenation status by a polarographic needle electrode. Acta Oncol. 33, 383–389 (1994).
Becker, A. et al. Oxygenation of squamous cell carcinoma of the head and neck: comparison of primary tumors, neck node metastases, and normal tissue. Int. J. Radiat. Oncol. Biol. Phys. 42, 35–41 (1998).
Le, Q. T. et al. Comparison of the comet assay and the oxygen microelectrode for measuring tumor oxygenation in head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 56, 375–383 (2003).
Vaupel, P., Briest, S. & Hockel, M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien. Med. Wochenschr. 152, 334–342 (2002).
Koong, A. C. et al. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 48, 919–922 (2000).
Lyng, H., Sundfor, K. & Rofstad, E. K. Oxygen tension in human tumours measured with polarographic needle electrodes and its relationship to vascular density, necrosis and hypoxia. Radiother. Oncol. 44, 163–169 (1997).
Fyles, A. W. et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother. Oncol. 48, 149–156 (1998).
Nordsmark, M. et al. Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas. Radiother. Oncol. 67, 35–44 (2003).
Movsas, B. et al. Hypoxia in human prostate carcinoma: an Eppendorf PO2 study. Am. J. Clin. Oncol. 24, 458–461 (2001).
Brizel, D. M. et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 56, 5347–5350 (1996).
Nordsmark, M. et al. The relationship between tumor oxygenation and cell proliferation in human soft tissue sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 35, 701–708 (1996).
Acknowledgements
The authors work is funded by grants from the United States National Institutes of Health (J.M.B. and W.R.W.) and the Health Research Council of New Zealand (W.R.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. Martin Brown has a research grant from Sanofi-Synthelabo, the company that owns tirapazamine.
J. Martin Brown and William R. Wilson have equity (<5%) in a company, Proacta Therapeutics Ltd., formed to exploit hypoxia in cancer treatment.
Related links
Related links
DATABASES
Cancer.gov
Entrez Gene
Glossary
- HYPOXIA
-
A low oxygen level. However, this means different levels to different investigators depending on the phenomenon being investigated. For the radiation biologist, hypoxia occurs at levels that produce severe radiation resistance or levels less than 0.1% O2 in the gas phase. Other effects of hypoxia occur at oxygen levels above and below this value.
- P-GLYCOPROTEIN
-
A protein localized to the cell membrane that actively pumps many drugs out of the cell. High levels of this protein lead to resistance to many anticancer drugs.
- PRODRUG
-
A latent form of a drug that can be activated by metabolism or other chemical transformation in the body.
- TOPOISOMERASE II
-
An enzyme that catalyses changes in DNA topology by transiently cleaving and re-ligating both strands of the double helix. This enzyme catalyses the passage of one DNA double-stranded molecule through another.
- ELECTROPHILE
-
A chemical group that reacts with electron-rich centres in molecules.
- FREE RADICAL
-
A compound with an unpaired electron and that is usually very reactive because of this feature.
- BYSTANDER EFFECT
-
Influence of a drug on untargeted cells, in the present context by diffusion of an activated cytotoxin from hypoxic cells to surrounding cells at higher oxygen concentrations.
- NITROGEN MUSTARD
-
DNA-crosslinking alkylating agents containing a bis(X-ethyl)amine group, where X is an electrophile that can react with nucleophiles such as the N7 position of guanine.
- GDEPT
-
(Gene-directed enzyme prodrug therapy). A cancer treatment strategy that aims to deliver a prodrug-activating enzyme specifically to tumour cells using gene therapy. The anticancer effect would be achieved by subsequent systemic administration of the non-toxic prodrug, which would be converted to a toxic drug preferentially in the tumour cells.
- CDEPT
-
(Clostridial-dependent enzyme prodrug therapy). A cancer therapy using the non-pathogenic species of the obligate anaerobe genus clostridia that have been genetically engineered to express a prodrug-activating enzyme. This is used to activate a prodrug within the hypoxic/necrotic regions that are colononized by the bacterium.
- ADEPT
-
(Antibody-directed enzyme prodrug therapy). A cancer treatment strategy that involves conjugation of a prodrug-activating enzyme (such as cytosine deaminase, which converts the non-toxic prodrug 5-fluorocytosine to the anticancer drug 5-fluorouracil) to a tumour-targeting antibody.
- VASCULAR-TARGETING AGENT
-
Drugs that damage existing blood vessels and therefore interfere with blood flow in tumours.
Rights and permissions
About this article
Cite this article
Brown, J., Wilson, W. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4, 437–447 (2004). https://doi.org/10.1038/nrc1367
Issue Date:
DOI: https://doi.org/10.1038/nrc1367
This article is cited by
-
A light-activatable theranostic combination for ratiometric hypoxia imaging and oxygen-deprived drug activity enhancement
Nature Communications (2024)
-
Exploring chronic and transient tumor hypoxia for predicting the efficacy of hypoxia-activated pro-drugs
npj Systems Biology and Applications (2024)
-
Glycolytic enzymes in non-glycolytic web: functional analysis of the key players
Cell Biochemistry and Biophysics (2024)
-
Intratumoural microbiota: from theory to clinical application
Cell Communication and Signaling (2023)
-
Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy
Journal of Nanobiotechnology (2023)