Key Points
-
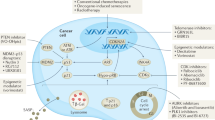
Oncogene-driven hyperproliferation and failsafe defects are the key cellular insults that enable malignant growth.
-
Mitogenic oncogenes preferentially provoke either apoptosis or premature senescence as cellular counteraction, leading to malignancies that harbour failsafe defects as 'oncogenic signatures'.
-
The 'lifelines' of cancer reflect defects in cellular growth control that are essential for tumorigenesis, such as disrupted apoptosis or senescence.By contrast, not all the mutations that are acquired during tumour progression are required to maintain neoplastic growth.
-
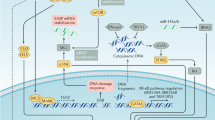
DNA-damaging anticancer drugs recruit the cell's failsafe machinery to execute apoptosis or cellular senescence.Defects in stress-response programmes that are acquired during tumour formation can co-select for drug resistance — even prior to drug exposure.
-
Identifying therapeutic approaches to 'cut the lifelines of cancer' is an appealing concept.By contrast, utilization or restoration of drug effector programmes that are not targeted during tumour formation could be a more efficient alternative.
-
Large-scale genetic screens and physiological test systems that allow high-throughput functional genomics are needed to identify key defects in failsafe pathways and to validate their implication for drug responses and novel therapeutic interventions.
Abstract
Apoptosis and senescence are cellular failsafe programmes that counteract excessive mitogenic signalling from activated oncogenes. Cancellation of apoptosis or senescence is therefore a prerequisite for tumour formation, and the ability of the cancer cell to disrupt these processes can be considered its 'lifeline'. Ironically, the efficacy of anticancer agents also depends on the activation of apoptosis or an acutely inducible form of cellular senescence. Understanding how the 'lifelines' of the cancer cell interfere with treatment sensitivity is of crucial importance for developing safer and more effective treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane, D. P. Cancer: p53, guardian of the genome. Nature 358, 15–16 (1992).
Levine, A. J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Schmitt, C. A. et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1, 289–298 (2002). Apoptosis is the sole p53 effector function selected against during MYC -driven lymphomagenesis, whereas chromosomal instability or checkpoint defects merely occurred as byproducts of p53 loss.
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Kiyono, T. et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396, 84–88 (1998).
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999). The first report about the creation of a human tumour cell by introduction of telomerase, an SV40 large-T-containing genomic fragment and oncogenic HRAS into normal human epithelial and fibroblast cells.
Seger, Y. R. et al. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2, 401–413 (2002). Introduction of telomerase is dispensable for the transformation of a human cell by two cooperating oncogenes — E1A and oncogenic HRAS — in combination with MDM2.
Schmitt, C. A. et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002). Premature senescence serves as a drug-responsive programme in vivo that improves treatment outcome when apoptosis is blocked. Tumours harbouring defects in senescence-controlling genes such as p53 or INK4A display impaired treatment responses.
Rothe, M., Sarma, V., Dixit, V. M. & Goeddel, D. V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 269, 1424–1427 (1995).
Lee, S. Y., Kandala, G., Liou, M. L., Liou, H. C. & Choi, Y. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc. Natl Acad. Sci. USA 93, 9699–9703 (1996).
Zong, W. X., Edelstein, L. C., Chen, C., Bash, J. & Gelinas, C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 13, 382–387 (1999).
Klefstrom, J. et al. Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-kappaB activation. EMBO J. 16, 7382–7392 (1997).
Mayo, M. W. et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278, 1812–1815 (1997).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Wotherspoon, A. C. et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 (1993).
Hermine, O. et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N. Engl. J. Med. 347, 89–94 (2002).
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Huettner, C. S., Zhang, P., Van Etten, R. A. & Tenen, D. G. Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nature Genet. 24, 57–60 (2000).
Jain, M. et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104 (2002).
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002). Using regulatable oncogene constructs, references 17–21 show that oncogenic signalling is not only required to initiate, but also to maintain, the malignant phenotype.
Emmerich, F. et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood 94, 3129–3134 (1999).
Willis, T. G. et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96, 35–45 (1999).
Bargou, R. C. et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J. Clin. Invest. 100, 2961–2969 (1997).
Davis, R. E., Brown, K. D., Siebenlist, U. & Staudt, L. M. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 194, 1861–1874 (2001).
Lucas, P. C. et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J. Biol. Chem. 276, 19012–19019 (2001).
Wang, C. Y., Cusack, J. C. Jr, Liu, R. & Baldwin, A. S. Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nature Med. 5, 412–417 (1999).
Liu, H. et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 122, 1286–1294 (2002).
Neubauer, A., He, M., Schmidt, C. A., Huhn, D. & Liu, E. T. Genetic alterations in the p53 gene in the blast crisis of chronic myelogenous leukemia: analysis by polymerase chain reaction based techniques. Leukemia 7, 593–600 (1993).
Du, M., Peng, H., Singh, N., Isaacson, P. G. & Pan, L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood 86, 4587–4593 (1995).
Sill, H., Goldman, J. M. & Cross, N. C. Homozygous deletions of the p16 tumor-suppressor gene are associated with lymphoid transformation of chronic myeloid leukemia. Blood 85, 2013–2016 (1995).
Neumeister, P. et al. Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas. Gastroenterology 112, 1871–1875 (1997).
Reznikoff, C. A. et al. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 56, 2886–2890 (1996). Establishes the model of INK4A-mediated senescence as an oncogene-provoked failsafe mechanism that is disrupted in manifest malignancies.
Yeager, T. R. et al. Overcoming cellular senescence in human cancer pathogenesis. Genes Dev. 12, 163–174 (1998).
Lin, A. W. & Lowe, S. W. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl Acad. Sci. USA 98, 5025–5030 (2001).
Hiyama, T. et al. Helicobacter pylori eradication therapy for high-grade mucosa-associated lymphoid tissue lymphomas of the stomach with analysis of p53 and K-ras alteration and microsatellite instability. Int. J. Oncol. 18, 1207–1212 (2001).
Wright, W. E. & Shay, J. W. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Med. 6, 849–851 (2000).
Blasco, M. A., Rizen, M., Greider, C. W. & Hanahan, D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nature Genet. 12, 200–204 (1996).
Land, H., Parada, L. F. & Weinberg, R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304, 596–602 (1983).
Leone, G., DeGregori, J., Sears, R., Jakoi, L. & Nevins, J. R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387, 422–426 (1997).
Lin, H. J., Eviner, V., Prendergast, G. C. & White, E. Activated H-ras rescues E1A-induced apoptosis and cooperates with E1A to overcome p53-dependent growth arrest. Mol. Cell. Biol. 15, 4536–4544 (1995).
Alevizopoulos, K., Vlach, J., Hennecke, S. & Amati, B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 16, 5322–5333 (1997).
Kauffmann-Zeh, A. et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385, 544–548 (1997).
Hermeking, H. et al. Identification of CDK4 as a target of c-MYC. Proc. Natl Acad. Sci. USA 97, 2229–2234 (2000).
Seoane, J., Le, H. V. & Massague, J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 (2002).
Desai, K. V. et al. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc. Natl Acad. Sci. USA 99, 6967–6972 (2002).
Serrano, M., Gomez-Lahoz, E., DePinho, R. A., Beach, D. & Bar-Sagi, D. Inhibition of Ras-induced proliferation and cellular transformation by p16INK4. Science 267, 249–252 (1995).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997). Activation of oncogenic RAS acutely induces a INK4A-associated senescent phenotype.
Peeper, D. S. et al. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 386, 177–181 (1997).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14, 3037–3050 (2000).
Rane, S. G., Cosenza, S. C., Mettus, R. V. & Reddy, E. P. Germ line transmission of the Cdk4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell. Biol. 22, 644–656 (2002). References 50 and 51 provide genetic evidence that senescence is a INK4A/RB-controlled programme as cells that overexpress a INK4A-insensitive CDK4 mutant, as well as knockout cells that lack all three RB-family members, fail to arrest.
Chin, L. et al. Cooperative effects of INK4a and Ras in melanoma susceptibility in vivo. Genes Dev. 11, 2822–2834 (1997).
Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. The oncogene and Polycomb-group gene Bmi-1 regulates cell proliferation and senescence through the Ink4a locus. Nature 397, 164–168 (1999).
Brookes, S. et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21, 2936–2945 (2002).
Huot, T. J. et al. Biallelic mutations in p16(INK4a) confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol. Cell. Biol. 22, 8135–8143 (2002).
Lazarov, M. et al. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nature Med. 8, 1105–1114 (2002).
Zou, X. et al. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 16, 2923–2934 (2002).
Schutte, M. et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 57, 3126–3130 (1997). Comprehensive work showing that virtually all pancreatic carcinomas display defects in the INK4A/RB pathway.
Sherr, C. J. The INK4a/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001).
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2, 103–112 (2002).
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Palmero, I., Pantoja, C. & Serrano, M. p19ARF links the tumour suppressor p53 to Ras. Nature 395, 125–126 (1998).
Peeper, D. S. et al. A functional screen identifies hDRIL1 as an oncogene that rescues RAS-induced senescence. Nature Cell Biol. 4, 148–153 (2002).
Zindy, F. et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12, 2424–2433 (1998). Proposes the ARF–p53 axis as the failsafe mechanism that counteracts MYC siganalling by apoptosis induction.
Hermeking, H. & Eick, D. Mediation of c-Myc-induced apoptosis by p53. Science 265, 2091–2093 (1994).
Evan, G. I. et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992).
Santoni-Rugiu, E. et al. E2F activity is essential for survival of Myc-overexpressing human cancer cells. Oncogene 21, 6498–6509 (2002).
Rudolph, B. et al. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 15, 3065–3076 (1996).
Hsu, B. et al. Evidence that c-myc mediated apoptosis does not require wild-type p53 during lymphomagenesis. Oncogene 11, 175–179 (1995).
Eischen, C. M., Weber, J. D., Roussel, M. F., Sherr, C. J. & Cleveland, J. L. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13, 2658–2669 (1999).
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999).
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348, 331–333 (1990).
Eischen, C. M., Roussel, M. F., Korsmeyer, S. J. & Cleveland, J. L. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol. Cell. Biol. 21, 7653–7662 (2001).
Krimpenfort, P., Quon, K. C., Mooi, W. J., Loonstra, A. & Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413, 83–86 (2001).
Ruas, M. & Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378, F115–F177 (1998).
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001).
Bardeesy, N. et al. Dual inactivation of RB and p53 pathways in RAS-induced melanomas. Mol. Cell. Biol. 21, 2144–2153 (2001).
Weber, J. D. et al. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 14, 2358–2365 (2000).
Preudhomme, C. et al. Clinical significance of p53 mutations in newly diagnosed Burkitt's lymphoma and acute lymphoblastic leukemia: a report of 48 cases. J. Clin. Oncol. 13, 812–820 (1995).
Lindstrom, M. S., Klangby, U. & Wiman, K. G. p14ARF homozygous deletion or MDM2 overexpression in Burkitt lymphoma lines carrying wild type p53. Oncogene 20, 2171–2177 (2001).
Imamura, J. et al. Mutation of the p53 gene in neuroblastoma and its relationship with N-myc amplification. Cancer Res. 53, 4053–4058 (1993).
Gazzeri, S. et al. p53 genetic abnormalities and myc activation in human lung carcinoma. Int. J. Cancer 58, 24–32 (1994).
Yu, Q., He, M., Lee, N. H. & Liu, E. T. Identification of Myc-mediated death response pathways by microarray analysis. J. Biol. Chem. 277, 13059–13066 (2002).
Nowak, M. A. et al. The role of chromosomal instability in tumor initiation. Proc. Natl Acad. Sci. USA 99, 16226–16231 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Lowe, S. W., Ruley, H. E., Jacks, T. & Housman, D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74, 957–967 (1993).
Lowe, S. W. et al. p53 status and the efficacy of cancer therapy in vivo. Science 266, 807–810 (1994).
Dohner, H. et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 85, 1580–1589 (1995). References 86–88 underscore the impact of the TP53 gene status on the outcome of anticancer therapy in preclinical and clinical settings.
Schmitt, C. A. & Lowe, S. W. Apoptosis and therapy. J. Pathol. 187, 127–137 (1999).
Chang, B. D. et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18, 4808–4818 (1999).
te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002). Provides evidence that neoadjuvant chemotherapy can cause senescence in human epithelial tumours in vivo , which is associated with wild-type p53 status and elevated INK4A protein levels.
Maloney, K. W., McGavran, L., Odom, L. F. & Hunger, S. P. Acquisition of p16(INK4A) and p15(INK4B) gene abnormalities between initial diagnosis and relapse in children with acute lymphoblastic leukemia. Blood 93, 2380–2385 (1999).
Carter, T. L. et al. Hemizygous p16(INK4A) deletion in pediatric acute lymphoblastic leukemia predicts independent risk of relapse. Blood 97, 572–574 (2001).
Sander, C. A. et al. p53 mutation is associated with progression in follicular lymphomas. Blood 82, 1994–2004 (1993).
Elenitoba-Johnson, K. S. et al. Homozygous deletions at chromosome 9p21 involving p16 and p15 are associated with histologic progression in follicle center lymphoma. Blood 91, 4677–4685 (1998).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Murphy, J. F., McGregor, J. L. & Leung, L. L. Senescent human neutrophil binding to thrombospondin (TSP): evidence for a TSP-independent pathway of phagocytosis by macrophages. Br. J. Haematol. 102, 957–964 (1998).
Hansen, M. H., Nielsen, H. & Ditzel, H. J. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc. Natl Acad. Sci. USA 98, 12659–12664 (2001).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001). Senescent human fibroblasts might act as 'feeder' cells by stimulating premalignant and malignant epithelial cells to proliferate in culture and to form tumours in mice.
Waldman, T. et al. Cell-cycle arrest versus cell death in cancer therapy. Nature Med. 3, 1034–1036 (1997).
Mudry, R. E., Fortney, J. E., York, T., Hall, B. M. & Gibson, L. F. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood 96, 1926–1932 (2000).
Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress Bcl2 is involved. Cancer Res. 55, 2284–2292 (1995).
Soni, R. et al. Selective in vivo and in vitro effects of a small molecule inhibitor of cyclin-dependent kinase 4. J. Natl Cancer Inst. 93, 436–446 (2001).
Schuler, M. et al. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J. Clin. Oncol. 19, 1750–1758 (2001).
Hayflick, L. & Moorhead, P. S. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Sherr, C. J. & DePinho, R. A. Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410 (2000).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995). First evidence for an age-dependent increase in senescent cells in skin samples from human donors, hereby linking cellular senescence to organismic ageing.
Shelton, D. N., Chang, E., Whittier, P. S., Choi, D. & Funk, W. D. Microarray analysis of replicative senescence. Curr. Biol. 9, 939–945 (1999).
Dimri, G. P., Itahana, K., Acosta, M. & Campisi, J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol. Cell. Biol. 20, 273–285 (2000).
Harvey, M. et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8, 2457–2467 (1993).
Ferbeyre, G. et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14, 2015–2027 (2000).
Pearson, M. et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207–210 (2000).
Petropoulou, C., Trougakos, I. P., Kolettas, E., Toussaint, O. & Gonos, E. S. Clusterin/apolipoprotein J is a novel biomarker of cellular senescence that does not affect the proliferative capacity of human diploid fibroblasts. FEBS Lett. 509, 287–297 (2001).
Comi, P., Chiaramonte, R. & Maier, J. A. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp. Cell Res. 219, 304–308 (1995).
Pantoja, C. & Serrano, M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene 18, 4974–4982 (1999).
Chang, B. D. et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 59, 3761–3767 (1999). Shows that differentiation-inducing anticancer agents such as retinoic acid can cause a senescence-like growth-arrest phenotype in a breast cancer xenotransplant model.
Chang, B. D. et al. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl Acad. Sci. USA 99, 389–394 (2002).
Dumont, P. et al. Growth kinetics rather than stress accelerate telomere shortening in cultures of human diploid fibroblasts in oxidative stress-induced premature senescence. FEBS Lett. 502, 109–112 (2001).
Ramirez, R. et al. Massive telomere loss is an early event of DNA damage-induced apoptosis. J. Biol. Chem. 278, 836–842 (2003).
Acknowledgements
Research in the author's laboratory is supported by grants from the Deutsche Forschungsgemeinschaft and Deutsche Krebshilfe. The author is a Special Fellow of the Leukemia & Lymphoma Society. He is grateful for stimulating discussions with B. Dörken, and critical comments to the manuscript by S. Lee.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
FURTHER INFORMATION
Glossary
- FAILSAFE PROGRAMMES
-
Stress-responsive, genetically encoded programmes that ultimately eliminate defective or inappropriately proliferating cells from the cell cycle to ensure cellular integrity.
- CYTOSTASIS
-
An acute, stress-inducible, long-term cell-cycle arrest that displays characteristics of cellular senescence.
Rights and permissions
About this article
Cite this article
Schmitt, C. Senescence, apoptosis and therapy — cutting the lifelines of cancer. Nat Rev Cancer 3, 286–295 (2003). https://doi.org/10.1038/nrc1044
Issue Date:
DOI: https://doi.org/10.1038/nrc1044
This article is cited by
-
Drug repurposing—an emerging strategy in cancer therapeutics
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)
-
Sunitinib facilitates metastatic breast cancer spreading by inducing endothelial cell senescence
Breast Cancer Research (2020)
-
H3K9me3-mediated epigenetic regulation of senescence in mice predicts outcome of lymphoma patients
Nature Communications (2020)
-
Oncogene-induced senescence mediated by c-Myc requires USP10 dependent deubiquitination and stabilization of p14ARF
Cell Death & Differentiation (2018)
-
Reversible senescence of human colon cancer cells after blockage of mitosis/cytokinesis caused by the CNF1 cyclomodulin from Escherichia coli
Scientific Reports (2018)