Abstract
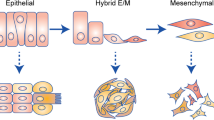
During cancer progression, malignant cells in the tumour invade surrounding tissues. This transformation of adherent cells to a motile phenotype has been associated with the epithelial–mesenchymal transition (EMT). Here, we show that EMT-activated cells migrate through micropillar arrays as a collectively advancing front that scatters individual cells. Individual cells with few neighbours dispersed with fast, straight trajectories, whereas cells that encountered many neighbours migrated collectively with epithelial biomarkers. We modelled these emergent dynamics using a physical analogy to phase transitions during binary-mixture solidification, and validated it using drug perturbations, which revealed that individually migrating cells exhibit diminished chemosensitivity. Our measurements also indicate a degree of phenotypic plasticity as cells interconvert between individual and collective migration. The study of multicellular behaviours with single-cell resolution should enable further quantitative insights into heterogeneous tumour invasion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fidler, I. J. & Hart, I. R. Biological diversity in metastatic neoplasms: Origins and implications. Science 217, 998–1003 (1982).
Willis, R. A. The Spread of Tumours in The Human Body 3rd edn (Butterworths, 1973).
Thiery, J. P., Acloque, H., Huang, R. Y. J. & Nieto, M. A. Epithelial–mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nature Rev. Cancer 9, 265–273 (2009).
Yilmaz, M. & Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 28, 15–33 (2009).
Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010).
Friedl, P., Locker, J., Sahai, E. & Segall, J. E. Classifying collective cancer cell invasion. Nature Cell Biol. 14, 777–783 (2012).
Polacheck, W. J., Zervantonakis, I. K. & Kamm, R. D. Tumor cell migration in complex microenvironments. Cell. Mol. Life Sci. 70, 1335–1356 (2013).
Thompson, E. W., Newgreen, D. F. & Tarin, D. Carcinoma invasion and metastasis: A role for epithelial–mesenchymal transition? Cancer Res. 65, 5991–5995 (2005).
Wang, W. et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 62, 6278–6288 (2002).
Alexander, S., Koehl, G. E., Hirschberg, M., Geissler, E. K. & Friedl, P. Dynamic imaging of cancer growth and invasion: A modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147–1154 (2008).
Giampieri, S. et al. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nature Cell Biol. 11, 1287–1296 (2009).
Simpson, K. J. et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nature Cell Biol. 10, 1027–1038 (2008).
Vitorino, P. & Meyer, T. Modular control of endothelial sheet migration. Genes Dev. 22, 3268–3281 (2008).
Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 115, 453–466 (1962).
Farooqui, R. & Fenteany, G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell Sci. 118, 51–63 (2005).
Poujade, M. et al. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl Acad. Sci. USA 104, 15988–15993 (2007).
Ng, M. R., Besser, A., Danuser, G. & Brugge, J. S. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J. Cell Biol. 199, 545–563 (2012).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nature Mater. 10, 469–475 (2011).
Bindschadler, M. & McGrath, J. L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 120, 876–884 (2007).
Nnetu, K. D., Knorr, M., Strehle, D., Zink, M. & Käs, J. A. Directed persistent motion maintains sheet integrity during multi-cellular spreading and migration. Soft Matter 8, 6913–6921 (2012).
Irimia, D. Microfluidic technologies for temporal perturbations of chemotaxis. Annu. Rev. Biomed. Eng. 12, 259–284 (2010).
Pathak, A. & Kumar, S. Biophysical regulation of tumor cell invasion: Moving beyond matrix stiffness. Integr. Biol. 3, 267–278 (2011).
Cano, A. et al. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000).
Zhou, B. P. et al. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nature Cell Biol. 6, 931–940 (2004).
Javaid, S. et al. Dynamic chromatin modification sustains epithelial–mesenchymal transition following inducible expression of Snail-1. Cell Rep. 5, 1679–1689 (2013).
Overholtzer, M. et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl Acad. Sci. USA 103, 12405–12410 (2006).
Altschuler, S. J. & Wu, L. F. Cellular heterogeneity: Do differences make a difference? Cell 141, 559–563 (2010).
Irimia, D. & Toner, M. Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr. Biol. 1, 506–512 (2009).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nature Methods 5, 695–702 (2008).
Hung, W. C. et al. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J. Cell Biol. 202, 807–824 (2013).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA 109, 10334–10339 (2012).
Wolf, K. et al. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069–1084 (2013).
Worster, M. G. Solidification of an alloy from a cooled boundary. J. Fluid Mech. 167, 481–501 (1986).
Smolen, G. A. et al. A genome-wide RNAi screen identifies multiple RSK-dependent regulators of cell migration. Genes Dev. 24, 2654–2665 (2010).
Cohen, M. S., Zhang, C., Shokat, K. M. & Taunton, J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 308, 1318–1321 (2005).
Brabletz, T. To differentiate or not–routes towards metastasis. Nature Rev. Cancer 12, 425–436 (2012).
Balzer, E. M. et al. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 26, 4045–4056 (2012).
Steinberg, M. S. Differential adhesion in morphogenesis: A modern view. Curr. Opin. Genet. Dev. 17, 281–286 (2007).
Worster, M. G. Convection in mushy layers. Annu. Rev. Fluid Mech. 29, 91–122 (1997).
Guiot, C., Pugno, N., Delsanto, P. P. & Deisboeck, T. S. Physical aspects of cancer invasion. Phys. Biol. 4, 1–6 (2007).
Anjum, R. & Blenis, J. The RSK family of kinases: Emerging roles in cellular signalling. Nature Rev. Mol. Cell Biol. 9, 747–758 (2008).
de Rooij, J., Kerstens, A., Danuser, G., Schwartz, M. A. & Waterman-Storer, C. M. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 171, 153–164 (2005).
Mark, S. et al. Physical model of the dynamic instability in an expanding cell culture. Biophys. J. 98, 361–370 (2010).
Rolli, C. G. et al. Switchable adhesive substrates: Revealing geometry dependence in collective cell behavior. Biomaterials 33, 2409–2418 (2012).
Nunes, J. K., Tsai, S. S. H., Wan, J. & Stone, H. A. Dripping and jetting in microfluidic multiphase flows applied to particle and fiber synthesis. J. Phys. D 46, 114002 (2013).
Rolli, C. G., Seufferlein, T., Kemkemer, R. & Spatz, J. P. Impact of tumor cell cytoskeleton organization on invasiveness and migration: A microchannel-based approach. PLoS ONE 5, e8726 (2010).
Sharma, S. V., Haber, D. A. & Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nature Rev. Cancer 10, 241–253 (2010).
Acknowledgements
We thank J. Taunton for the gift of FMK-MEA, A. Besser and G. Danuser for assistance with cell tracking, and O. Hurtado for assistance with fabrication. This work was supported by the Merck Fellowship of the Damon Runyon Cancer Research Foundation (DRG-2065-10) to I.Y.W., the Howard Hughes Medical Institute to D.A.H., as well as the National Institutes of Health under CA129933 to D.A.H., EB002503 (BioMEMS Resource Center) to M.T., and CA135601 and GM092804 to D.I.
Author information
Authors and Affiliations
Contributions
D.A.H. and D.I. conceived the project; I.Y.W., S.J., D.A.H., M.T. and D.I. designed the research; I.Y.W., S.J. and E.A.W. performed the experiments; I.Y.W. and E.A.W. performed image analysis; I.Y.W., E.A.W. and S.P. performed data analysis; I.Y.W. and M.T. developed the theoretical model; I.Y.W., M.T. and D.I. wrote the manuscript with feedback from S.J., E.A.W., S.P. and D.A.H. All authors reviewed the data and final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 8907 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 1604 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 1947 kb)
Supplementary Movie 3
Supplementary Movie 3 (MOV 1875 kb)
Supplementary Movie 4
Supplementary Movie 4 (MOV 1993 kb)
Rights and permissions
About this article
Cite this article
Wong, I., Javaid, S., Wong, E. et al. Collective and individual migration following the epithelial–mesenchymal transition. Nature Mater 13, 1063–1071 (2014). https://doi.org/10.1038/nmat4062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4062
This article is cited by
-
High-throughput and proteome-wide discovery of endogenous biomolecular condensates
Nature Chemistry (2024)
-
Single-copy Snail upregulation causes partial epithelial-mesenchymal transition in colon cancer cells
BMC Cancer (2023)
-
Microfluidic devices for precise measurements of cell directionality reveal a role for glutamine during cell migration
Scientific Reports (2023)
-
Geometric constraint-triggered collagen expression mediates bacterial-host adhesion
Nature Communications (2023)
-
2D profiling of tumor chemotactic and molecular phenotype at single cell resolution using a SERS-microfluidic chip
Nano Research (2022)