Abstract
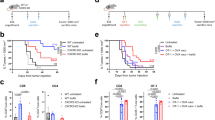
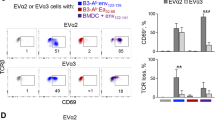
To understand why cancer vaccine–induced T cells often do not eradicate tumors, we studied immune responses in mice vaccinated with gp100 melanoma peptide in incomplete Freund's adjuvant (peptide/IFA), which is commonly used in clinical cancer vaccine trials. Peptide/IFA vaccination primed tumor-specific CD8+ T cells, which accumulated not in tumors but rather at the persisting, antigen-rich vaccination site. Once there, primed T cells became dysfunctional and underwent antigen-driven, interferon-γ (IFN-γ)- and Fas ligand (FasL)-mediated apoptosis, resulting in hyporesponsiveness to subsequent vaccination. Provision of CD40-specific antibody, Toll-like receptor 7 (TLR7) agonist and interleukin-2 (IL-2) reduced T cell apoptosis but did not prevent vaccination-site sequestration. A nonpersisting vaccine formulation shifted T cell localization toward tumors, inducing superior antitumor activity while reducing systemic T cell dysfunction and promoting memory formation. These data show that persisting vaccine depots can induce specific T cell sequestration, dysfunction and deletion at vaccination sites; short-lived formulations may overcome these limitations and result in greater therapeutic efficacy of peptide-based cancer vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
27 September 2013
In the version of this article initially published, the author list omitted coauthor Ryan T. Sowell and his contribution. The author list and author contributions have been corrected in the HTML and PDF versions of the article.
References
Schwartzentruber, D.J. et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 364, 2119–2127 (2011).
Kantoff, P.W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Kenter, G.G. et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361, 1838–1847 (2009).
Schuster, S.J. et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J. Clin. Oncol. 29, 2787–2794 (2011).
Lizeé, G. et al. Harnessing the power of the immune system to target cancer. Annu. Rev. Med. 64, 71–90 (2013).
Rosenberg, S.A. et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 175, 6169–6176 (2005).
Rosenberg, S.A., Yang, J.C. & Restifo, N.P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Smyth, M.J., Dunn, G.P. & Schreiber, R.D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90, 1–50 (2006).
Lizee, G., Radvanyi, L.G., Overwijk, W.W. & Hwu, P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin. Cancer Res. 12, 4794–4803 (2006).
Bonhoure, F. & Gaucheron, J. Montanide ISA 51 VG as adjuvant for human vaccines. J. Immunother. 29, 647–648 (2006).
Reinhardt, R.L., Bullard, D.C., Weaver, C.T. & Jenkins, M.K. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J. Exp. Med. 197, 751–762 (2003).
Redmond, W.L. & Sherman, L.A. Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284 (2005).
Aichele, P., Brduscha-Riem, K., Zinkernagel, R.M., Hengartner, H. & Pircher, H. T cell priming versus T cell tolerance induced by synthetic peptides. J. Exp. Med. 182, 261–266 (1995).
Toes, R.E., Offringa, R., Blom, R.J., Melief, C.J. & Kast, W.M. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc. Natl. Acad. Sci. USA 93, 7855–7860 (1996).
Bijker, M.S. et al. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur. J. Immunol. 38, 1033–1042 (2008).
Overwijk, W.W. et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 188, 277–286 (1998).
Overwijk, W.W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Overwijk, W.W. et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J. Immunol. 176, 5213–5222 (2006).
Bijker, M.S. et al. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 179, 5033–5040 (2007).
Smith, R.T. & Bridges, R.A. Immunological unresponsiveness in rabbits produced by neonatal injection of defined antigens. J. Exp. Med. 108, 227–250 (1958).
Critchfield, J.M. et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science 263, 1139–1143 (1994).
Rabinovich, B.A. et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc. Natl. Acad. Sci. USA 105, 14342–14346 (2008).
Janicki, C.N., Jenkinson, S.R., Williams, N.A. & Morgan, D.J. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 68, 2993–3000 (2008).
Riquelme, E., Carreno, L.J., Gonzalez, P.A. & Kalergis, A.M. The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity. Eur. J. Immunol. 39, 2259–2269 (2009).
Zehn, D., Lee, S.Y. & Bevan, M.J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Muraoka, D. et al. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J. Immunol. 185, 3768–3776 (2010).
Refaeli, Y., Van Parijs, L., Alexander, S.I. & Abbas, A.K. Interferon gamma is required for activation-induced death of T lymphocytes. J. Exp. Med. 196, 999–1005 (2002).
Gabrilovich, D.I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Ugel, S. et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2, 628–639 (2012).
van Heijst, J.W.J. et al. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science 325, 1265–1269 (2009).
Schoenberger, S.P., Toes, R.E., van der Voort, E.I., Offringa, R. & Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Kedl, R.M. et al. CD40 stimulation accelerates deletion of tumor-specific CD8+ T cells in the absence of tumor-antigen vaccination. Proc. Natl. Acad. Sci. USA 98, 10811–10816 (2001).
Ahonen, C.L. et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199, 775–784 (2004).
Ly, L.V. et al. Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res. 70, 8339–8346 (2010).
Verdeil, G., Marquardt, K., Surh, C.D. & Sherman, L.A. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc. Natl. Acad. Sci. USA 105, 16683–16688 (2008).
Wojciechowski, S. et al. Bim mediates apoptosis of CD127lo effector T cells and limits T cell memory. Eur. J. Immunol. 36, 1694–1706 (2006).
Wojciechowski, S. et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J. Exp. Med. 204, 1665–1675 (2007).
Hildeman, D.A. et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity 16, 759–767 (2002).
Matsushita, H. et al. Cancer exome analysis reveals a T-cell–dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
Gajewski, T.F., Fuertes, M., Spaapen, R., Zheng, Y. & Kline, J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 23, 286–292 (2011).
Wherry, E.J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Jin, H.T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 107, 14733–14738 (2010).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Wherry, E.J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
den Boer, A.T. et al. Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J. Immunol. 167, 2522–2528 (2001).
den Boer, A.T. et al. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J. Immunol. 172, 6074–6079 (2004).
Rezvani, K. et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica 96, 432–440 (2011).
Speiser, D.E. & Romero, P. Toward improved immunocompetence of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1467–1469 (2005).
Appay, V. et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J. Immunol. 177, 1670–1678 (2006).
Kenter, G.G. et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 14, 169–177 (2008).
Speetjens, F.M. et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin. Cancer Res. 15, 1086–1095 (2009).
Leffers, N. et al. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int. J. Cancer 125, 2104–2113 (2009).
Yamshchikov, G.V. et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int. J. Cancer 92, 703–711 (2001).
Schaefer, J.T. et al. Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. J. Transl. Med. 8, 79 (2010).
Graham, B.S. et al. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One 5, e11995 (2010).
Ma, W. et al. Effect of long-term storage in TRIzol on microarray-based gene expression profiling. Cancer Epidemiol. Biomarkers Prev. 19, 2445–2452 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B 57, 289–300 (1995).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Jakt, L.M., Cao, L., Cheah, K.S. & Smith, D.K. Assessing clusters and motifs from gene expression data. Genome Res. 11, 112–123 (2001).
Acknowledgements
The authors thank G. Lizee, N. Martin-Orozco and J. Khalili for their helpful comments on this manuscript. This work was supported by the National Institutes of Health (NIH) grants R01 1CA143077 (W.W.O.) and P01 CA128913 (P.H. and W.W.O.) and a Melanoma Research Alliance Established Investigator Award (W.W.O.).
Author information
Authors and Affiliations
Contributions
Y.H. designed and performed experiments and wrote the manuscript. Z.D., N.J., Y.Y., M.A.M., X.-F.H., N.R.G., G.N., S.M.D.-E., W.P., C.L. and Y.L. performed experiments. R.T.S. and K.S.S. constructed VSV.gp100. B.R. and P.H. provided the v-effLuc-GFP–expressing retroviral construct and imaging expertise. Z.W., W.M. and R.E.D. performed and analyzed gene expression arrays. W.W.O. conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–19 and Supplementary Results (PDF 933 kb)
Rights and permissions
About this article
Cite this article
Hailemichael, Y., Dai, Z., Jaffarzad, N. et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 19, 465–472 (2013). https://doi.org/10.1038/nm.3105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3105
This article is cited by
-
CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies
Cellular & Molecular Immunology (2022)
-
Identification of HLA-A2 restricted epitopes of glypican-3 and induction of CTL responses in HLA-A2 transgenic mice
Cancer Immunology, Immunotherapy (2022)
-
Evaluation of the efficacy and safety of TAS0313 in adults with recurrent glioblastoma
Cancer Immunology, Immunotherapy (2022)
-
Mechanism of action of DSP-7888 (adegramotide/nelatimotide) Emulsion, a peptide-based therapeutic cancer vaccine with the potential to turn up the heat on non-immunoreactive tumors
Clinical and Translational Oncology (2022)
-
Designing spatial and temporal control of vaccine responses
Nature Reviews Materials (2021)