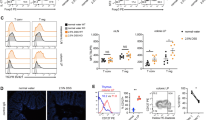
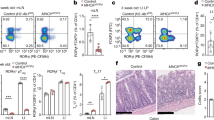
Abstract
The intestinal epithelium functions to absorb nutrients and to protect the organism against microbes. To prevent autoimmune attack on this vital tissue, T cell tolerance to intestinal self-antigens must be established. Central tolerance mechanisms involve medullary thymic epithelial cells (mTECs), which use endogenously expressed peripheral-tissue antigens (PTAs) to delete self-reactive thymocytes. The prevailing model for the induction of peripheral tolerance involves cross-presentation of tissue antigens by quiescent dendritic cells. Here we show that lymph node stromal cells present endogenously expressed PTAs to T cells. Moreover, antigen presentation by lymph node stroma is sufficient to induce primary activation and subsequent tolerance among CD8+ T cells. Thus, lymph node stromal cells are functionally akin to mTECs and provide a direct strategy for purging the peripheral repertoire of self-reactive T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kyewski, B. & Klein, L. A central role for central tolerance. Annu. Rev. Immunol. 24, 571–606 (2006).
Gallegos, A.M. & Bevan, M.J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200, 1039–1049 (2004).
Gallegos, A.M. & Bevan, M.J. Central tolerance: good but imperfect. Immunol. Rev. 209, 290–296 (2006).
Klein, L. & Kyewski, B. Self-antigen presentation by thymic stromal cells: a subtle division of labor. Curr. Opin. Immunol. 12, 179–186 (2000).
Redmond, W.L. & Sherman, L.A. Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284 (2005).
Heath, W.R. & Carbone, F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Steinman, R.M. et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. NY Acad. Sci. 987, 15–25 (2003).
Kurts, C. et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 (1996).
Adler, A.J. et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 187, 1555–1564 (1998).
Morgan, D.J. et al. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc. Natl. Acad. Sci. USA 96, 3854–3858 (1999).
Belz, G.T. et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002).
Vezys, V., Olson, S. & Lefrancois, L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity 12, 505–514 (2000).
Mayerova, D., Parke, E.A., Bursch, L.S., Odumade, O.A. & Hogquist, K.A. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity 21, 391–400 (2004).
Azukizawa, H. et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 33, 1879–1888 (2003).
Scheinecker, C., McHugh, R., Shevach, E.M. & Germain, R.N. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196, 1079–1090 (2002).
Kelsall, B.L. & Leon, F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol. Rev. 206, 132–148 (2005).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212, 256–271 (2006).
Lefrancois, L. & Puddington, L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu. Rev. Immunol. 24, 681–704 (2006).
Vermaelen, K.Y., Carro-Muino, I., Lambrecht, B.N. & Pauwels, R.A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 193, 51–60 (2001).
Turley, S., Poirot, L., Hattori, M., Benoist, C. & Mathis, D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 198, 1527–1537 (2003).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
van Stipdonk, M.J., Lemmens, E.E. & Schoenberger, S.P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 423–429 (2001).
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
Bevan, M.J. & Fink, P.J. The CD8 response on autopilot. Nat. Immunol. 2, 381–382 (2001).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000).
Arnold, B. Parenchymal cells in immune and tolerance induction. Immunol. Lett. 89, 225–228 (2003).
Staton, T.L. et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat. Immunol. 7, 482–488 (2006).
Cose, S., Brammer, C., Khanna, K.M., Masopust, D. & Lefrancois, L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 36, 1423–1433 (2006).
Johnstone, C.N. et al. Characterization of mouse A33 antigen, a definitive marker for basolateral surfaces of intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G500–G510 (2000).
Abud, H.E., Johnstone, C.N., Tebbutt, N.C. & Heath, J.K. The murine A33 antigen is expressed at two distinct sites during development, the ICM of the blastocyst and the intestinal epithelium. Mech. Dev. 98, 111–114 (2000).
Anderson, M.S. et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005).
Anderson, M.S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2, 1032–1039 (2001).
Maemura, K. et al. Antigen-presenting cells expressing glutamate decarboxylase 67 were identified as epithelial cells in glutamate decarboxylase 67-GFP knock-in mouse thymus. Tissue Antigens 67, 198–206 (2006).
Hecht, N.B. The making of a spermatozoon: a molecular perspective. Dev. Genet. 16, 95–103 (1995).
Garcia, C.A. et al. Dendritic cells in human thymus and periphery display a proinsulin epitope in a transcription-dependent, capture-independent fashion. J. Immunol. 175, 2111–2122 (2005).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E.S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Steinman, R.M. The control of immunity and tolerance by dendritic cell. Pathol. Biol. (Paris) 51, 59–60 (2003).
Heath, W.R., Kurts, C., Miller, J.F. & Carbone, F.R. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 187, 1549–1553 (1998).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367 (2001).
Vallon-Eberhard, A., Landsman, L., Yogev, N., Verrier, B. & Jung, S. Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol. 176, 2465–2469 (2006).
Huang, F.P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Macpherson, A.J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Turley, S.J., Lee, J-W., Dutton-Swain, N., Mathis, D. & Benoist, C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc. Natl. Acad. Sci. USA 102, 17729–17733 (2005).
Acknowledgements
We thank M. Nussenzweig (Rockefeller University) and L. Lefrancois (University of Connecticut) for the gifts of CD11c-EYFP transgenic mice and iFABP-tOVA transgenic mice, respectively; W. Heath, G. Losyev, K. Irving, A. Bellemare-Pelletier and M. Werneck for technical advice or support; and C. Benoist, K. Wucherpfennig and A. Goldrath for critically reading the manuscript. Supported by the Claudia Adams Barr Program for Innovative Cancer Research, the Diabetes and Endocrinology Research Center of the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK36836-19 to S.J.T.) and the Institut National de la Recherche Agronomique (M.E.).
Author information
Authors and Affiliations
Contributions
J.-W.L. and S.J.T. contributed to every aspect of this manuscript (experimentation, mouse work, writing and figure composition); S.J.T. provided most of the funding; M.E. did the cell trafficking experiments; J.S. did the in vitro presentation assays with purified IECs; J.E.B. did the immunoblots; A.C.C. and A.Y. helped with immunofluorescence staining of tissue sections; and J.K.H. contributed reagents and guidance on experiments involving the A33 antigen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
T cell proliferation in peripheral lymphoid tissues is not due to the direct presentation of OVA antigen by IECs. (PDF 2641 kb)
Supplementary Fig. 2
Analysis of bone marrow chimerism. (PDF 712 kb)
Supplementary Fig. 3
Analysis of A33 antigen expression in parenchymal tissues by fluorescence microscopy. (PDF 153 kb)
Supplementary Table 1
Primer sequences. (PDF 646 kb)
Rights and permissions
About this article
Cite this article
Lee, JW., Epardaud, M., Sun, J. et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol 8, 181–190 (2007). https://doi.org/10.1038/ni1427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1427
This article is cited by
-
The aging of the immune system and its implications for transplantation
GeroScience (2023)
-
Programmable multistage drug delivery to lymph nodes
Nature Nanotechnology (2020)
-
Lymph node stromal cells: cartographers of the immune system
Nature Immunology (2020)
-
Tissue-Engineered Stromal Reticula to Study Lymph Node Fibroblastic Reticular Cells in Type I Diabetes
Cellular and Molecular Bioengineering (2020)
-
Roadmap to Local Tumour Growth: Insights from Cervical Cancer
Scientific Reports (2019)