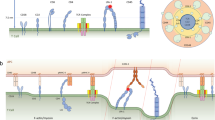
Abstract
T cell receptor (TCR) engagement leads to actin polymerization at the site of T cell contact with antigen-presenting cells. Here we have studied the dynamic activity of proteins involved in regulating actin polymerization in live T cells after activation. Two such adaptor proteins, Nck and the Wiskott-Aldrich syndrome protein (WASp), were recruited to the TCR during initial T cell activation, where they colocalized with the tyrosine kinase Zap70. The recruitment of Nck and WASp depended on TCR-induced tyrosine phosphorylation and the LAT and SLP-76 adaptors. Nck and WASp migrated peripherally and accumulated at an actin-rich circumferential ring. Thus, actin polymerization regulated by the TCR begins at the TCR. Molecules recruited to the TCR regulate actin polymerization and this process drives plasma membrane movement and cellular spreading.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jordan, M.S., Singer, A.L. & Koretzky, G.A. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4, 110–116 (2003).
Samelson, L.E. Signal transduction mediated by the T cell antigen receptor: the role of adaptor proteins. Annu. Rev. Immunol. 20, 371–394 (2002).
Bunnell, S.C., Kapoor, V., Trible, R.P., Zhang, W. & Samelson, L.E. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14, 315–329 (2001).
Finkelstein, L.D. & Schwartzberg, P.L. Tec kinases: shaping T-cell activation through actin. Trends Cell Biol. 14, 443–451 (2004).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Burack, W.R., Lee, K.H., Holdorf, A.D., Dustin, M.L. & Shaw, A.S. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 169, 2837–2841 (2002).
Sasahara, Y. et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell 10, 1269–1281 (2002).
Zeng, R. et al. SLP-76 Coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact Site. J. Immunol. 171, 1360–1368 (2003).
Lee, K.-H. et al. T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Acuto, O. & Cantrell, D. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18, 165–184 (2000).
Dustin, M.L. & Cooper, J.A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1, 23–29 (2000).
Bromley, S.K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Derry, J.M., Ochs, H.D. & Francke, U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 79, 922 (1994).
Remold-O'Donnell, E., Rosen, F.S. & Kenney, D.M. Defects in Wiskott-Aldrich syndrome blood cells. Blood 87, 2621–2631 (1996).
Gallego, M.D., Santamaria, M., Pena, J. & Molina, I.J. Defective actin reorganization and polymerization of Wiskott-Aldrich T cells in response to CD3-mediated stimulation. Blood 90, 3089–3097 (1997).
Molina, I.J., Sancho, J., Terhorst, C., Rosen, F.S. & Remold-O'Donnell, E. T cells of patients with the Wiskott-Aldrich syndrome have a restricted defect in proliferative responses. J. Immunol. 151, 4383–4390 (1993).
Cannon, J.L. et al. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity 15, 249–259 (2001).
Badour, K., Zhang, J. & Siminovitch, K.A. The Wiskott-Aldrich syndrome protein: forging the link between actin and cell activation. Immunol. Rev. 192, 98–112 (2003).
Badour, K. et al. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112 (2004).
Cory, G.O., Cramer, R., Blanchoin, L. & Ridley, A.J. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell 11, 1229–1239 (2003).
Zhang, W., Sloan-Lancaster, J., Kitchen, J., Trible, R.P. & Samelson, L.E. LAT: the Zap 70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92, 83–92 (1998).
Zhang, W. et al. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275, 23355–23361 (2000).
Liu, S.K., Fang, N., Koretzky, G.A. & McGlade, C.J. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9, 67–75 (1999).
Finco, T.S., Kadlecek, T., Zhang, W., Samelson, L.E. & Weiss, A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 9, 617–626 (1998).
Bubeck Wardenburg, J. et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9, 607–616 (1998).
Wunderlich, L., Farago, A., Downward, J. & Buday, L. Association of Nck with tyrosine-phosphorylated SLP-76 in activated T lymphocytes. Eur. J. Immunol. 29, 1068–1075 (1999).
Rivero-Lezcano, O., Marcilla, A., Sameshima, J. & Robbins, K. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol. Cell. Biol. 15, 5725–5731 (1995).
Krause, M. et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 (2000).
Cannon, J.L. & Burkhardt, J.K. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol. Rev. 186, 90–99 (2002).
Gil, D., Schamel, W.W., Montoya, M., Sanchez-Madrid, F. & Alarcon, B. Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109, 901–912 (2002).
Snapper, S.B. & Rosen, F.S. The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu. Rev. Immunol. 17, 905–929 (1999).
Thrasher, A.J. WASp in immune-system organization and function. Nat. Rev. Immunol. 2, 635–646 (2002).
Badour, K. et al. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity 18, 141–154 (2003).
Bunnell, S.C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Szollosi, J., Damjanovich, S. & Matyus, L. Application of fluorescence resonance energy transfer in the clinical laboratory: routine and research. Cytometry 34, 159–179 (1998).
Seminario, M.C. & Wange, R.L. Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial. Immunol. Rev. 192, 80–97 (2003).
Bonello, G. et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 117, 1009–1016 (2004).
Rivero-Lezcano, O.M., Marcilla, A., Sameshima, J.H. & Robbins, K.C. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol. Cell. Biol. 15, 5725–5731 (1995).
Kupfer, A. & Kupfer, H. Imaging immune cell interactions and functions: SMACs and the immunological synapse. Semin. Immunol. 15, 295–300 (2003).
Krawczyk, C. et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343 (2002).
Zacharias, D.A., Violin, J.D., Newton, A.C. & Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Shan, X. et al. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell. Biol. 20, 6945–6957 (2000).
Astoul, E. et al. Approaches to define antigen receptor-induced serine kinase signal transduction pathways. J. Biol. Chem. 278, 9267–9275 (2003).
Laurence, A., Astoul, E., Hanrahan, S., Totty, N. & Cantrell, D. Identification of pro-interleukin 16 as a novel target of MAP kinases in activated T lymphocytes. Eur. J. Immunol. 34, 587–597 (2004).
Acknowledgements
The authors thank S. Saitoh and D. Mahadeo for their help; S. Garfield and S. Wincovitch for their help in the operation of the LSM 510; B. Taylor for cell sorting; and P. Schwartzberg and C. Sommers for reading the manuscript. Supported by the Cancer Research Institute (S.C.B.) and the Office of Research on Women's Health, Foundation for Advanced Education in the Sciences, National Institutes of Health (R.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Known and proposed molecular interactions leading to TCR-induced actin polymerization. (PDF 64 kb)
Supplementary Fig. 2
FRET analysis controls. (PDF 72 kb)
Supplementary Fig. 3
Reconstitution of Jurkat cells with the lipid phosphatase PTEN. (PDF 177 kb)
Supplementary Video 1
Dynamic localization of WASp-YFP actin-CFP in live activated T cells. (MOV 431 kb)
Supplementary Video 2
Dynamic localization of SLP-76-YFP WASp-CFP in live activated T cells. (MOV 735 kb)
Supplementary Video 3
Dynamic localization of SLP-76-YFP CFP-Nck in live activated T cells. (MOV 5311 kb)
Supplementary Video 4
Dynamic localization of YFP-Nck WASp-CFP in live activated T cells. (MOV 660 kb)
Rights and permissions
About this article
Cite this article
Barda-Saad, M., Braiman, A., Titerence, R. et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 6, 80–89 (2005). https://doi.org/10.1038/ni1143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1143
This article is cited by
-
Dysfunctional natural killer cells can be reprogrammed to regain anti-tumor activity
The EMBO Journal (2024)
-
Phase separation in immune signalling
Nature Reviews Immunology (2022)
-
Targeting the actin nucleation promoting factor WASp provides a therapeutic approach for hematopoietic malignancies
Nature Communications (2021)
-
TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps
Nature Communications (2019)
-
Mechanosensing through immunoreceptors
Nature Immunology (2019)