Abstract
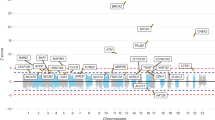
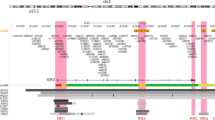
Familial clustering studies indicate that breast cancer risk has a substantial genetic component1,2,3. To identify new breast cancer risk variants, we genotyped approximately 300,000 SNPs in 1,600 Icelandic individuals with breast cancer and 11,563 controls using the Illumina Hap300 platform. We then tested selected SNPs in five replication sample sets. Overall, we studied 4,554 affected individuals and 17,577 controls. Two SNPs consistently associated with breast cancer: ∼25% of individuals of European descent are homozygous for allele A of rs13387042 on chromosome 2q35 and have an estimated 1.44-fold greater risk than noncarriers, and for allele T of rs3803662 on 16q12, about 7% are homozygous and have a 1.64-fold greater risk. Risk from both alleles was confined to estrogen receptor–positive tumors. At present, no genes have been identified in the linkage disequilibrium block containing rs13387042. rs3803662 is near the 5′ end of TNRC9 , a high mobility group chromatin–associated protein whose expression is implicated in breast cancer metastasis to bone4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Amundadottir, L.T. et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 1, e65 (2004).
Lichtenstein, P. et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85 (2000).
Cannon-Albright, L.A. et al. Familiality of cancer in Utah. Cancer Res. 54, 2378–2385 (1994).
Smid, M. et al. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 24, 2261–2267 (2006).
Easton, D.F. How many more breast cancer predisposition genes are there? Breast Cancer Res. 1, 14–17 (1999).
Balmain, A., Gray, J. & Ponder, B. The genetics and genomics of cancer. Nat. Genet. 33 Suppl., 238–244 (2003).
Pharoah, P.D. et al. Polygenic susceptibility to breast cancer and implications for prevention. Nat. Genet. 31, 33–36 (2002).
Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J. Natl. Cancer Inst. 98, 1382–1396 (2006).
Cox, A. et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat. Genet. 39, 352–358 (2007).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Lohmueller, K.E., Pearce, C.L., Pike, M., Lander, E.S. & Hirschhorn, J.N. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat. Genet. 33, 177–182 (2003).
Trikalinos, T.A., Ntzani, E.E., Contopoulos-Ioannidis, D.G. & Ioannidis, J.P. Establishment of genetic associations for complex diseases is independent of early study findings. Eur. J. Hum. Genet. 12, 762–769 (2004).
Skol, A.D., Scott, L.J., Abecasis, G.R. & Boehnke, M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat. Genet. 38, 209–213 (2006).
Johannsson, O.T. et al. Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur. J. Cancer 33, 362–371 (1997).
Honrado, E., Benitez, J. & Palacios, J. Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit. Rev. Oncol. Hematol. 59, 27–39 (2006).
McVean, G.A. et al. The fine-scale structure of recombination rate variation in the human genome. Science 304, 581–584 (2004).
Winckler, W. et al. Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308, 107–111 (2005).
Rakha, E.A., Green, A.R., Powe, D.G., Roylance, R. & Ellis, I.O. Chromosome 16 tumor-suppressor genes in breast cancer. Genes Chromosom. Cancer 45, 527–535 (2006).
James, J.J. et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br. J. Cancer 89, 660–665 (2003).
Koenders, P.G. et al. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res. Treat. 18, 27–32 (1991).
Barrett, J.C. & Cardon, L.R. Evaluating coverage of genome-wide association studies. Nat. Genet. 38, 659–662 (2006).
Kutyavin, I.V. et al. A novel endonuclease IV post-PCR genotyping system. Nucleic Acids Res. 34, e128 (2006).
Gretarsdottir, S. et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat. Genet. 35, 131–138 (2003).
Grant, S.F. et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38, 320–323 (2006).
Acknowledgements
We thank all participants for taking part in this research. We thank S. Sveinsdottir and K. Alexiusdottir for assistance in recruitment of patients and collection of clinical information. This project was funded in part by contract number 018827 (POLYGENE) from the 6th Framework Program of the European Union, the Swedish Cancer Society, the Gustav V Jubilee Foundation, the Bert von Kantzow Foundation and the Nilsson-Ehle Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Simon N Stacey, Andrei Manolescu, Patrick Sulem, Thorunn Rafnar, Julius Gudmundsson, Sigurjon A Gudjonsson, Gisli Masson, Margret Jakobsdottir, Steinunn Thorlacius, Agnar Helgason, Magali Mouy, Jona Saemundsdottir, Valgerdur M Backman, Larus Gudmundsson, Kristleifur Kristjansson, Jon T Bergthorsson, Jelena Kostic, Michael L Frigge, Frank Geller, Daniel Gudbjartsson, Jeffrey R Gulcher, Unnur Thorsteinsdottir, Augustine Kong and Kari Stefansson are employees and/or shareholders of deCODE genetics.
Supplementary information
Supplementary Fig. 1
Q-Q plot for 311,524 SNPs. (PDF 156 kb)
Supplementary Table 1
Association results for SNPs that did not replicate significantly outside Iceland. (PDF 43 kb)
Supplementary Table 2
Comparison of recently diagnosed and long-term survivor groups of Icelandic breast cancer patients. (PDF 52 kb)
Supplementary Table 3
Swedish Familial and consecutively recruited breast cancer patient groups. (PDF 38 kb)
Supplementary Table 4
Odds ratios for A-rs13387042 and T-rs3803662 in carriers and noncarriers of the Icelandic BRCA2 999del5 mutation. (PDF 58 kb)
Supplementary Table 5
Primers used in Centaurus SNP and PCR assays. (PDF 65 kb)
Rights and permissions
About this article
Cite this article
Stacey, S., Manolescu, A., Sulem, P. et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor–positive breast cancer. Nat Genet 39, 865–869 (2007). https://doi.org/10.1038/ng2064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng2064
This article is cited by
-
2q35-rs13387042 variant and the risk of breast cancer: a case–control study
Molecular Biology Reports (2022)
-
Penalized partial least squares for pleiotropy
BMC Bioinformatics (2021)
-
The prevalence of ataxia telangiectasia mutated (ATM) variants in patients with breast cancer patients: a systematic review and meta-analysis
Cancer Cell International (2021)
-
The Multi-Faced Role of PAPP-A in Post-Partum Breast Cancer: IGF-Signaling is Only the Beginning
Journal of Mammary Gland Biology and Neoplasia (2020)
-
Low-penetrance susceptibility variants and postmenopausal oestrogen receptor positive breast cancer
Journal of Genetics (2020)