Abstract
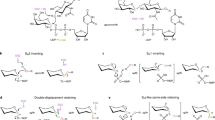
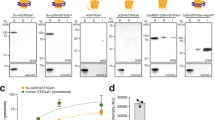
Glycosyltransferases are ubiquitous enzymes that catalyze the assembly of glycoconjugates throughout all kingdoms of nature. A long-standing problem is the rational design of probes that can be used to manipulate glycosyltransferase activity in cells and tissues. Here we describe the rational design and synthesis of a nucleotide sugar analog that inhibits, with high potency both in vitro and in cells, the human glycosyltransferase responsible for the reversible post-translational modification of nucleocytoplasmic proteins with O-linked N-acetylglucosamine residues (O-GlcNAc). We show that the enzymes of the hexosamine biosynthetic pathway can transform, both in vitro and in cells, a synthetic carbohydrate precursor into the nucleotide sugar analog. Treatment of cells with the precursor lowers O-GlcNAc in a targeted manner with a single-digit micromolar EC50. This approach to inhibition of glycosyltransferases should be applicable to other members of this superfamily of enzymes and enable their manipulation in a biological setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Varki, A. & Lowe, J.B. Biological roles of glycans. in Essentials of Glycobiology (eds. Varki, A. et al.) (CSH Press, 2009).
Platt, F.M., Neises, G.R., Dwek, R.A. & Butters, T.D. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 269, 8362–8365 (1994).
Lowe, J.B. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 15, 531–538 (2003).
Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 (2000).
Hart, G.W., Housley, M.P. & Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007).
Roquemore, E.P., Chevrier, M.R., Cotter, R.J. & Hart, G.W. Dynamic O-GlcNAcylation of the small heat shock protein α B-crystallin. Biochemistry 35, 3578–3586 (1996).
Yang, X. et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969 (2008).
Sinclair, D.A. et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc. Natl. Acad. Sci. USA 106, 13427–13432 (2009).
Zachara, N.E. et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 (2004).
Slawson, C. et al. Perturbations in O-linked β-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J. Biol. Chem. 280, 32944–32956 (2005).
Caldwell, S.A. et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29, 2831–2842 (2010).
Yuzwa, S.A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 (2008).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G.W. & Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 101, 10804–10809 (2004).
Liu, J., Marchase, R.B. & Chatham, J.C. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am. J. Physiol. Heart Circ. Physiol. 293, H1391–H1399 (2007).
Ngoh, G.A., Hamid, T., Prabhu, S.D. & Jones, S.P. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 297, H1711–H1719 (2009).
Lyons, S.D., Sant, M.E. & Christopherson, R.I. Cytotoxic mechanisms of glutamine antagonists in mouse L1210 leukemia. J. Biol. Chem. 265, 11377–11381 (1990).
Lenzen, S. & Panten, U. Alloxan: history and mechanism of action. Diabetologia 31, 337–342 (1988).
Gross, B.J., Kraybill, B.C. & Walker, S. Discovery of O-GlcNAc transferase inhibitors. J. Am. Chem. Soc. 127, 14588–14589 (2005).
Ngoh, G.A., Watson, L.J., Facundo, H.T., Dillmann, W. & Jones, S.P. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J. Mol. Cell. Cardiol. 45, 313–325 (2008).
Keppler, O.T., Horstkorte, R., Pawlita, M., Schmidt, C. & Reutter, W. Biochemical engineering of the N-acyl side chain of sialic acid: biological implications. Glycobiology 11, 11R–18R (2001).
Agard, N.J. & Bertozzi, C.R. Chemical approaches to perturb, profile, and perceive glycans. Acc. Chem. Res. 42, 788–797 (2009).
Jones, M.B. et al. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol. Bioeng. 85, 394–405 (2004).
Vocadlo, D.J., Hang, H.C., Kim, E.J., Hanover, J.A. & Bertozzi, C.R. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc. Natl. Acad. Sci. USA 100, 9116–9121 (2003).
Lairson, L.L., Henrissat, B., Davies, G.J. & Withers, S.G. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77, 521–555 (2008).
Yuasa, H., Izumi, M. & Hashimoto, H. Thiasugars: potential glycosidase inhibitors. Curr. Top. Med. Chem. 9, 76–86 (2009).
Tsuruta, O., Shinohara, G., Yuasa, H. & Hashimoto, H. UDP-N-acetyl-5-thio-galactosamine is a substrate of lactose synthase. Bioorg. Med. Chem. Lett. 7, 2523–2526 (1997).
Tsuruta, O. et al. Synthesis of GDP-5-thiosugars and their use as glycosyl donor substrates for glycosyltransferases. J. Org. Chem. 68, 6400–6406 (2003).
Offen, W. et al. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 25, 1396–1405 (2006).
Lambert, J.B. & Wharry, S.M. Conformational-analysis of 5-thio-D-glucose. J. Org. Chem. 46, 3193–3196 (1981).
Zhao, G., Guan, W., Cai, L. & Wang, P.G. Enzymatic route to preparative-scale synthesis of UDP-GlcNAc/GalNAc, their analogues and GDP-fucose. Nat. Protoc. 5, 636–646 (2010).
Tanahashi, E., Kiso, M. & Hasegawa, A. A facile synthesis of 2-acetamido-2-deoxy-5-thio-D-glucopyranose. Carbohydr. Res. 117, 304–308 (1983).
Davis, L.I. & Blobel, G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc. Natl. Acad. Sci. USA 84, 7552–7556 (1987).
Lubas, W.A., Smith, M., Starr, C.M. & Hanover, J.A. Analysis of nuclear pore protein p62 glycosylation. Biochemistry 34, 1686–1694 (1995).
Greig, I.R., Macauley, M.S., Williams, I.H. & Vocadlo, D.J. Probing synergy between two catalytic strategies in the glycoside hydrolase O-GlcNAcase using multiple linear free energy relationships. J. Am. Chem. Soc. 131, 13415–13422 (2009).
Martinez-Fleites, C. et al. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat. Struct. Mol. Biol. 15, 764–765 (2008).
Khidekel, N. et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat. Chem. Biol. 3, 339–348 (2007).
O'Donnell, N., Zachara, N.E., Hart, G.W. & Marth, J.D. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol. Cell. Biol. 24, 1680–1690 (2004).
Dennis, R.J. et al. Structure and mechanism of a bacterial β-glucosaminidase having O-GlcNAcase activity. Nat. Struct. Mol. Biol. 13, 365–371 (2006).
Csuk, R. & Glanzer, B.I. A short synthesis of 2-acetamido-2-deoxy-5-thio-D-glucose and D-mannose from 5-thio-glucal. J. Chem. Soc. Chem. Commun. 343–344 (1986).
Saxon, E. & Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Boehmelt, G. et al. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J. 19, 5092–5104 (2000).
Sarkar, A.K., Fritz, T.A., Taylor, W.H. & Esko, J.D. Disaccharide uptake and priming in animal cells: inhibition of sialyl Lewis X by acetylated Gal β 1→4GlcNAc β-O-naphthalenemethanol. Proc. Natl. Acad. Sci. USA 92, 3323–3327 (1995).
Ralton, J.E., Milne, K.G., Guther, M.L., Field, R.A. & Ferguson, M.A. The mechanism of inhibition of glycosylphosphatidylinositol anchor biosynthesis in Trypanosoma brucei by mannosamine. J. Biol. Chem. 268, 24183–24189 (1993).
Pesnot, T., Jorgensen, R., Palcic, M.M. & Wagner, G.K. Structural and mechanistic basis for a new mode of glycosyltransferase inhibition. Nat. Chem. Biol. 6, 321–323 (2010).
Lee, K.Y. et al. The hexapeptide inhibitor of Galβ1,3GalNAc-specific α2,3-sialyltransferase as a generic inhibitor of sialyltransferases. J. Biol. Chem. 277, 49341–49351 (2002).
Schneider, E.G., Nguyen, H.T. & Lennarz, W.J. The effect of tunicamycin, an inhibitor of protein glycosylation, on embryonic development in the sea urchin. J. Biol. Chem. 253, 2348–2355 (1978).
Hinderlich, S., Berger, M., Schwarzkopf, M., Effertz, K. & Reutter, W. Molecular cloning and characterization of murine and human N-acetylglucosamine kinase. Eur. J. Biochem. 267, 3301–3308 (2000).
Mio, T., Yamada-Okabe, T., Arisawa, M. & Yamada-Okabe, H. Functional cloning and mutational analysis of the human cDNA for phosphoacetylglucosamine mutase: identification of the amino acid residues essential for the catalysis. Biochim. Biophys. Acta 1492, 369–376 (2000).
Bourgeaux, V., Piller, F. & Piller, V. Two-step enzymatic synthesis of UDP-N-acetylgalactosamine. Bioorg. Med. Chem. Lett. 15, 5459–5462 (2005).
Macauley, M.S., Stubbs, K.A. & Vocadlo, D.J. O-GlcNAcase catalyzes cleavage of thioglycosides without general acid catalysis. J. Am. Chem. Soc. 127, 17202–17203 (2005).
Acknowledgements
The plasmid encoding GNK was a gift from M. Berger (Charite Universitatsmedizin Berlin) and S. Hinderlich (University of Applied Sciences, Berlin), and the plasmid encoding AGX1 was a gift from V. Piller (Centre de Recherche Scientifique, UPR 4301). Polyclonal antibody to OGA was a gift from G. Hart (John Hopkins University). EMEG32 cells were a gift from T. Mak (University of Toronto). We thank G. Davies (University of York) for the E. coli expression construct of BtGH84. T.M.G. is a Sir Henry Wellcome postdoctoral fellow and a Michael Smith for Health Research (MSFHR) trainee award holder. D.J.V. is a scholar of MSFHR and holds a Canada Research Chair in Chemical Glycobiology. We thank the Natural Sciences and Engineering Research Council of Canada and Simon Fraser University for funding support.
Author information
Authors and Affiliations
Contributions
T.M.G. and D.J.V. designed experiments. T.M.G., W.F.Z., J.E.H., D.L.S. and L.D. carried out experiments. T.M.G., W.F.Z., D.L.S. and D.J.V. analyzed results. T.M.G. and D.J.V wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Provisional patent applications covering this work have been filed.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Schemes 1–4 and Supplementary Figures 1–15 (PDF 2362 kb)
Rights and permissions
About this article
Cite this article
Gloster, T., Zandberg, W., Heinonen, J. et al. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol 7, 174–181 (2011). https://doi.org/10.1038/nchembio.520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.520
This article is cited by
-
O-GlcNAcylation: a pro-survival response to acute stress in the cardiovascular and central nervous systems
European Journal of Medical Research (2024)
-
O-GlcNAcylation determines the translational regulation and phase separation of YTHDF proteins
Nature Cell Biology (2023)
-
O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations
Nature Communications (2023)
-
O-GlcNAcylation: an important post-translational modification and a potential therapeutic target for cancer therapy
Molecular Medicine (2022)
-
Global O-GlcNAcylation changes impact desmin phosphorylation and its partition toward cytoskeleton in C2C12 skeletal muscle cells differentiated into myotubes
Scientific Reports (2022)